Infusion of Kupffer Cells Expanded in Vitro Ameliorated Liver Fibrosis in a Murine Model of Liver Injury
- PMID: 33784833
- PMCID: PMC8020097
- DOI: 10.1177/09636897211004090
Infusion of Kupffer Cells Expanded in Vitro Ameliorated Liver Fibrosis in a Murine Model of Liver Injury
Abstract
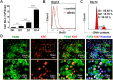
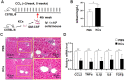
Transfer of exogenous macrophages represents an alternative technique to treat liver fibrosis. At present, bone marrow-derived monocytes and stem cells are the main sources for exogenous macrophages. Kupffer cells (KCs) are the resident macrophages in the liver and play a critical role in the liver homeostasis and diseases. It is unclear whether infusion of KCs can treat liver fibrosis. In this study, we observed that granulocyte-macrophage colony stimulating factor (GM-CSF) could improve the purity of cultured KCs and significantly up-regulate the expression of Cluster of Differentiation 11b (CD11b). The most important point is that GM-CSF could significantly promote the proliferation of KCs in vitro. KCs expanded in vitro still had the potential of M1/M2 polarization and phagocytosis. Furthermore, infusion of these KCs could ameliorate liver fibrosis induced by carbon tetrachloride (CCl4) in mice. Together, our results suggest that KCs are likely to be another source for macrophage therapy.
Keywords: GM-CSF; Kupffer cells; cell proliferation; cell therapy; liver fibrosis.
Conflict of interest statement
Figures



References
-
- Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2020;18(3):151–166. Online ahead of print. PMID: 33128017. - PubMed
-
- Ramachandran P, Iredale JP, Fallowfield JA. Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin Liver Dis. 2015;35(2):119–131. - PubMed
-
- Alison MR, Lin WR. Macrophages come on tap for liver fibrosis therapy. Hepatology. 2018;68(3):1194–1196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

