A multipurpose brachytherapy catheter to enable intratumoral injection
- PMID: 33785280
- PMCID: PMC8323107
- DOI: 10.1016/j.brachy.2020.10.012
A multipurpose brachytherapy catheter to enable intratumoral injection
Abstract
Purpose: To create and test a multipurpose brachytherapy catheter prototype enabling intratumoral injection and brachytherapy after a single catheter insertion.
Methods and materials: The design of the prototype consists of an outer tube and an inner syringe tube that can be filled with injectable agent. The outer sheath and inner syringe tube were constructed using polytetrafluoroethylene tubing, and the other components were 3D printed using dental resin and polylactic acid material. To demonstrate functionality, we injected in vitro phantoms with dyed saline. For proof of concept, we demonstrated the potential for the prototype to deliver cell therapy, enhance tumor delineation, deliver tattoo ink for pathology marking, avoid toxicity through local delivery of chemotherapy, and facilitate combination brachytherapy and immunotherapy.
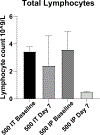
Results: The prototype enables accurate injection in vitro and in vivo without altering dosimetry. To illustrate the potential for delivery of cell therapies, we injected luciferase-expressing splenocytes and confirmed their delivery with bioluminescence imaging. To demonstrate feasibility of radiographically visualizing injected material, we delivered iohexol contrast intratumorally and confirmed tumor retention using Faxitron x-ray imaging. In addition, we show the potential of intratumoral administration to reduce toxicity associated with cyclophosphamide compared with systemic administration. To demonstrate feasibility, we treated tumor-bearing mice with brachytherapy (192Ir source, 2 Gy to 5 mm) in combination with intratumoral injection of 375,000 U of interleukin 2 and observed no increased toxicity.
Conclusions: These results demonstrate that a prototype multipurpose brachytherapy catheter enables accurate intratumoral injection and support the feasibility of combining intratumoral injection with brachytherapy.
Keywords: Brachytherapy; Cell therapy; Contrast-enhanced imaging; Interleukin 2; Intratumoral injection; Multipurpose brachytherapy catheter.
Copyright © 2021. Published by Elsevier Inc.
Figures




Similar articles
-
Brachytherapy with Intratumoral Injections of Radiometal-Labeled Polymers That Thermoresponsively Self-Aggregate in Tumor Tissues.J Nucl Med. 2017 Sep;58(9):1380-1385. doi: 10.2967/jnumed.117.189993. Epub 2017 Apr 13. J Nucl Med. 2017. PMID: 28408533
-
Evaluation of an active magnetic resonance tracking system for interstitial brachytherapy.Med Phys. 2015 Dec;42(12):7114-21. doi: 10.1118/1.4935535. Med Phys. 2015. PMID: 26632065 Free PMC article.
-
A 3D-printed patient-specific applicator guide for use in high-dose-rate interstitial brachytherapy for tongue cancer: a phantom study.Phys Med Biol. 2019 Jul 2;64(13):135002. doi: 10.1088/1361-6560/ab277e. Phys Med Biol. 2019. PMID: 31170698
-
ESTRO-ACROP guideline: Interstitial multi-catheter breast brachytherapy as Accelerated Partial Breast Irradiation alone or as boost - GEC-ESTRO Breast Cancer Working Group practical recommendations.Radiother Oncol. 2018 Sep;128(3):411-420. doi: 10.1016/j.radonc.2018.04.009. Epub 2018 Apr 21. Radiother Oncol. 2018. PMID: 29691075
-
[Endobronchial chemotherapy by direct injection of cytotoxic drugs into the tumor in lung cancer].Tuberk Toraks. 2008;56(3):337-43. Tuberk Toraks. 2008. PMID: 18932038 Review. Turkish.
Cited by
-
Intratumoral radiation dose heterogeneity augments antitumor immunity in mice and primes responses to checkpoint blockade.Sci Transl Med. 2024 Sep 18;16(765):eadk0642. doi: 10.1126/scitranslmed.adk0642. Epub 2024 Sep 18. Sci Transl Med. 2024. PMID: 39292804 Free PMC article.
-
Iodine-125 plesiotherapy for murine tumor treatment.Radiat Oncol. 2025 May 16;20(1):78. doi: 10.1186/s13014-025-02657-0. Radiat Oncol. 2025. PMID: 40380214 Free PMC article.
References
-
- Patel RB, Baniel CC, Sriramaneni RN, Bradley K, Markovina S, Morris ZS. Combining brachytherapy and immunotherapy to achieve in situ tumor vaccination: A review of cooperative mechanisms and clinical opportunities. Brachytherapy. 2018;17(6):995–1003. Epub 2018/08/02. doi: 10.1016/j.brachy.2018.07.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

