Comparison of five SARS-CoV-2 rapid antigen detection tests in a hospital setting and performance of one antigen assay in routine practice: a useful tool to guide isolation precautions?
- PMID: 33785377
- PMCID: PMC7999797
- DOI: 10.1016/j.jhin.2021.03.021
Comparison of five SARS-CoV-2 rapid antigen detection tests in a hospital setting and performance of one antigen assay in routine practice: a useful tool to guide isolation precautions?
Abstract
Background: In a hospital setting, there is a need for rapid detection of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to guide isolation measures and targeted admission.
Aim: To evaluate the diagnostic performance of five SARS-CoV-2 rapid nucleocapsid protein antigen detection (RAD) assays (Biosynex, Biotical, Orient Gene, Panbio and SD Biosensor), and describe the performance and impact of implementation of the SD Biosensor assay in an emergency department.
Methods: Sensitivity and specificity of the five RAD assays were analysed on 100 respiratory samples: 60 real-time reverse transcriptase polymerase chain reaction (rRT-PCR)-confirmed SARS-CoV-2-positive samples, 24 SARS-CoV-2 RNA-negative samples and 16 samples positive for other respiratory pathogens. The manufacturer's protocol was adapted to validate the antigen tests on transport media used for rRT-PCR in the authors' routine practice. The SD Biosensor RAD assay was implemented as a screening method for rapid diagnosis and targeted admission.
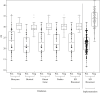
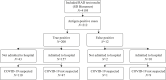
Findings: Sensitivity of the five RAD assays ranged from 88.9% to 100% for samples with cycle threshold values <26, and specificity ranged from 46.2% to 100%. During the implementation period, 4195 RAD tests were performed. Due to the rapid RAD result, 157 patients were transferred directly to the coronavirus disease 2019 (COVID-19) cohort ward instead of the regular ward (N=47) or the temporary COVID-19 ward (N=110).
Conclusion: The SD Biosensor, Biotical and Panbio SARS-CoV-2 antigen tests showed acceptable overall performance, and identified the majority of contagious patients. In the context of high prevalence of SARS-CoV-2, RAD tests can be used as a rapid screening tool to guide infection prevention measures and aid targeted admission.
Keywords: Infection prevention; Isolation precautions; Rapid antigen detection; SARS-CoV-2; rRT-PCR.
Copyright © 2021 The Healthcare Infection Society. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Lippi G., Simundic A.-M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020;58:1070–1076. - PubMed
-
- World Health Organization . WHO; Geneva: 2020. Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays.https://www.who.int/publications/i/item/antigen-detection-in-the-diagnos... Available at: [last accessed November 2020]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

