Inositol 1,4,5-trisphosphate 3-kinase B promotes Ca2+ mobilization and the inflammatory activity of dendritic cells
- PMID: 33785611
- PMCID: PMC8091591
- DOI: 10.1126/scisignal.aaz2120
Inositol 1,4,5-trisphosphate 3-kinase B promotes Ca2+ mobilization and the inflammatory activity of dendritic cells
Abstract
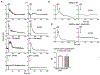
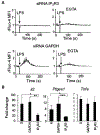
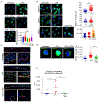
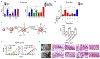
Innate immune responses to Gram-negative bacteria depend on the recognition of lipopolysaccharide (LPS) by a receptor complex that includes CD14 and TLR4. In dendritic cells (DCs), CD14 enhances the activation not only of TLR4 but also that of the NFAT family of transcription factors, which suppresses cell survival and promotes the production of inflammatory mediators. NFAT activation requires Ca2+ mobilization. In DCs, Ca2+ mobilization in response to LPS depends on phospholipase C γ2 (PLCγ2), which produces inositol 1,4,5-trisphosphate (IP3). Here, we showed that the IP3 receptor 3 (IP3R3) and ITPKB, a kinase that converts IP3 to inositol 1,3,4,5-tetrakisphosphate (IP4), were both necessary for Ca2+ mobilization and NFAT activation in mouse and human DCs. A pool of IP3R3 was located on the plasma membrane of DCs, where it colocalized with CD14 and ITPKB. Upon LPS binding to CD14, ITPKB was required for Ca2+ mobilization through plasma membrane-localized IP3R3 and for NFAT nuclear translocation. Pharmacological inhibition of ITPKB in mice reduced both LPS-induced tissue swelling and the severity of inflammatory arthritis to a similar extent as that induced by the inhibition of NFAT using nanoparticles that delivered an NFAT-inhibiting peptide specifically to phagocytic cells. Our results suggest that ITPKB may represent a promising target for anti-inflammatory therapies that aim to inhibit specific DC functions.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



Similar articles
-
CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation.Nature. 2009 Jul 9;460(7252):264-8. doi: 10.1038/nature08118. Epub 2009 Jun 14. Nature. 2009. PMID: 19525933
-
Effect of inositol 1,3,4,5-tetrakisphosphate on inositol trisphosphate-activated Ca2+ signaling in mouse lacrimal acinar cells.J Biol Chem. 1996 Mar 22;271(12):6766-70. doi: 10.1074/jbc.271.12.6766. J Biol Chem. 1996. PMID: 8636098
-
CD14 and NFAT mediate lipopolysaccharide-induced skin edema formation in mice.J Clin Invest. 2012 May;122(5):1747-57. doi: 10.1172/JCI60688. Epub 2012 Apr 2. J Clin Invest. 2012. PMID: 22466648 Free PMC article.
-
The dendritic cell life cycle.Cell Cycle. 2009 Dec;8(23):3816-21. doi: 10.4161/cc.8.23.9998. Epub 2009 Dec 4. Cell Cycle. 2009. PMID: 19887908 Review.
-
Regulation of B cell survival, development and function by inositol 1,4,5-trisphosphate 3-kinase B (Itpkb).Adv Enzyme Regul. 2011;51(1):66-73. doi: 10.1016/j.advenzreg.2010.08.001. Epub 2010 Oct 28. Adv Enzyme Regul. 2011. PMID: 21035494 Review.
Cited by
-
Specific immunosuppressive role of nanodrugs targeting calcineurin in innate myeloid cells.iScience. 2022 Aug 30;25(10):105042. doi: 10.1016/j.isci.2022.105042. eCollection 2022 Oct 21. iScience. 2022. PMID: 36124235 Free PMC article.
-
Ca2+ homeostasis: a potential target for cancer therapies.Biophys Rep. 2024 Oct 31;10(5):283-292. doi: 10.52601/bpr.2024.230023. Biophys Rep. 2024. PMID: 39539289 Free PMC article.
-
Saporin Toxin Delivered by Engineered Colloidal Nanoparticles Is Strongly Effective against Cancer Cells.Pharmaceutics. 2022 Jul 21;14(7):1517. doi: 10.3390/pharmaceutics14071517. Pharmaceutics. 2022. PMID: 35890411 Free PMC article.
-
Immunosuppression Affects Neutrophil Functions: Does Calcineurin-NFAT Signaling Matter?Front Immunol. 2021 Nov 2;12:770515. doi: 10.3389/fimmu.2021.770515. eCollection 2021. Front Immunol. 2021. PMID: 34795676 Free PMC article. Review.
-
An allergenic plant calmodulin from Artemisia pollen primes human DCs leads to Th2 polarization.Front Immunol. 2022 Sep 29;13:996427. doi: 10.3389/fimmu.2022.996427. eCollection 2022. Front Immunol. 2022. PMID: 36248805 Free PMC article.
References
-
- Herbst S, Shah A, Mazon Moya M, Marzola V, Jensen B, Reed A, Birrell MA, Saijo S, Mostowy S, Shaunak S, Armstrong-James D, Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol. Med . 7, 240–258 (2015). - PMC - PubMed
-
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, Costa B, Zaza A, Ricciardi-Castagnoli P, Granucci F, CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460, 264–268 (2009). - PubMed
-
- Goodridge HS, Simmons RM, Underhill DM, Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol . 178, 3107–3115 (2007). - PubMed
-
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, Moita LF, Taylor PR, Reis e Sousa C, Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med . 206, 2037–2051 (2009). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

