Cryptococcus neoformans - Infected Macrophages Release Proinflammatory Extracellular Vesicles: Insight into Their Components by Multi-omics
- PMID: 33785616
- PMCID: PMC8092229
- DOI: 10.1128/mBio.00279-21
Cryptococcus neoformans - Infected Macrophages Release Proinflammatory Extracellular Vesicles: Insight into Their Components by Multi-omics
Abstract
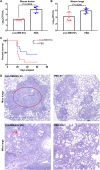
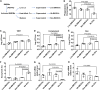
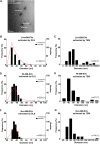
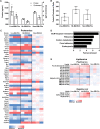
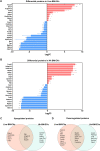
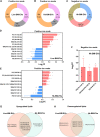
Cryptococcus neoformans causes deadly mycosis in immunocompromised individuals. Macrophages are key cells fighting against microbes. Extracellular vesicles (EVs) are cell-to-cell communication mediators. The roles of EVs from infected host cells in the interaction with Cryptococcus remain uninvestigated. Here, EVs from viable C. neoformans-infected macrophages reduced fungal burdens but led to shorter survival of infected mice. In vitro, EVs induced naive macrophages to an inflammatory phenotype. Transcriptome analysis showed that EVs from viable C. neoformans-infected macrophages activated immune-related pathways, including p53 in naive human and murine macrophages. Conserved analysis demonstrated that basic cell biological processes, including cell cycle and division, were activated by infection-derived EVs from both murine and human infected macrophages. Combined proteomics, lipidomics, and metabolomics of EVs from infected macrophages showed regulation of pathways such as extracellular matrix (ECM) receptors and phosphatidylcholine. This form of intermacrophage communication could serve to prepare cells at more distant sites of infection to resist C. neoformans infection.IMPORTANCECryptococcus neoformans causes cryptococcal meningitis, which is frequent in patients with HIV/AIDS, especially in less-developed countries. The incidence of cryptococcal meningitis is close to 1 million each year globally. Macrophages are key cells that protect the body against microbes, including C. neoformans Extracellular vesicles are a group of membrane structures that are released from cells such as macrophages that modulate cell activities via the transfer of materials such as proteins, lipids, and RNAs. In this study, we found that Cryptococcus neoformans-infected macrophages produce extracellular vesicles that enhance the inflammatory response in Cryptococcus-infected mice. These Cryptococcus neoformans-infected macrophage vesicles also showed higher fungicidal biological effects on inactivated macrophages. Using omics technology, unique protein and lipid signatures were identified in these extracellular vesicles. Transcriptome analysis showed that these vesicles activated immune-related pathways like p53 in naive macrophages. The understanding of this intermacrophage communication could provide potential targets for the design of therapeutic agents to fight this deadly mycosis.
Keywords: Cryptococcus neoformans; extracellular vesicles; lipidomics; metabolomics; proteomics.
Copyright © 2021 Zhang et al.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
