Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial
- PMID: 33785743
- PMCID: PMC8009873
- DOI: 10.1038/s41467-021-22177-1
Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial
Abstract
Type III interferons have been touted as promising therapeutics in outpatients with coronavirus disease 2019 (COVID-19). We conducted a randomized, single-blind, placebo-controlled trial (NCT04331899) in 120 outpatients with mild to moderate COVID-19 to determine whether a single, 180 mcg subcutaneous dose of Peginterferon Lambda-1a (Lambda) within 72 hours of diagnosis could shorten the duration of viral shedding (primary endpoint) or symptoms (secondary endpoint). In both the 60 patients receiving Lambda and 60 receiving placebo, the median time to cessation of viral shedding was 7 days (hazard ratio [HR] = 0.81; 95% confidence interval [CI] 0.56 to 1.19). Symptoms resolved in 8 and 9 days in Lambda and placebo, respectively, and symptom duration did not differ significantly between groups (HR 0.94; 95% CI 0.64 to 1.39). Both Lambda and placebo were well-tolerated, though liver transaminase elevations were more common in the Lambda vs. placebo arm (15/60 vs 5/60; p = 0.027). In this study, a single dose of subcutaneous Peginterferon Lambda-1a neither shortened the duration of SARS-CoV-2 viral shedding nor improved symptoms in outpatients with uncomplicated COVID-19.
Conflict of interest statement
C.H. and I.C. are scientists at Eiger BioPharmaceuticals, Inc., which provided the Interferon Lambda used for this study. J.G. serves on the board of Eiger BioPharmaceuticals, Inc. C.H. and I.C. own stock and options of Eiger BioPharmaceuticals, Inc. J.G. has an equity interest in Eiger BioPharmaceuticals, Inc. J.G. and I.C. are inventors on a pending patent application relating to the use of interferon lambda for coronavirus. Eiger BioPharmaceuticals played no role in study design, conduct of the study, or analysis of the data. All other authors declare no competing interests.
Figures
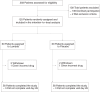
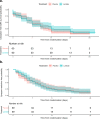
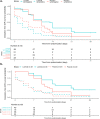
References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) (Johns Hopkins University, 2021). https://coronavirus.jhu.edu/map.html.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

