Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment
- PMID: 33785843
- PMCID: PMC8093220
- DOI: 10.1038/s41423-021-00655-2
Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment
Abstract
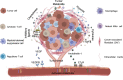
Chimeric antigen receptor (CAR)-T cell therapy has achieved successful outcomes against hematological malignancies and provided a new impetus for treating solid tumors. However, the efficacy of CAR-T cells for solid tumors remains unsatisfactory. The tumor microenvironment has an important role in interfering with and inhibiting the effector function of immune cells, among which upregulated inhibitory checkpoint receptors, soluble suppressive cytokines, altered chemokine expression profiles, aberrant vasculature, complicated stromal composition, hypoxia and abnormal tumor metabolism are major immunosuppressive mechanisms. In this review, we summarize the inhibitory factors that affect the function of CAR-T cells in tumor microenvironment and discuss approaches to improve CAR-T cell efficacy for solid tumor treatment by targeting those barriers.
Keywords: CAR-T; Microenvironment; Tumor.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Prasad V. Immunotherapy: Tisagenlecleucel - the first approved CAR-T-cell therapy: implications for payers and policy makers. Nat. Rev. Clin. Oncol. 2018;15:11–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

