Stem Cell Factor Neutralization Protects From Severe Anaphylaxis in a Murine Model of Food Allergy
- PMID: 33786039
- PMCID: PMC8005333
- DOI: 10.3389/fimmu.2021.604192
Stem Cell Factor Neutralization Protects From Severe Anaphylaxis in a Murine Model of Food Allergy
Abstract
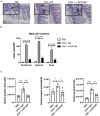
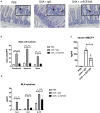
Food allergy is a growing public health problem with ~15 million people affected in the United States. In allergic food disease, IgE on mast cells bind to ingested antigens leading to the activation and degranulation of mast cells. Stem cell factor (SCF) is mast cell growth and activation factor that is required for peripheral tissue mast cells. We targeted a specific isoform of SCF, the larger 248 amino acid form, that drives peripheral tissue mast cell differentiation using a specific monoclonal antibody in a model of food allergy. Ovalbumin sensitized and intragastrically challenged mice were monitored for symptoms of anaphylaxis including respiratory distress, diarrhea, and a reduction in body temperature. During the second week of challenges, allergic mice were injected with an antibody to block SCF248 or given IgG control. Mice treated with α-SCF248 had a decreased incidence of diarrhea and no reduction in body temperature suggesting a reduction in anaphylaxis compared to IgG control treated animals. Re-stimulated mesenteric lymph nodes indicated that α-SCF248 treated mice had decreased OVA-specific Th2 cytokine production compared to IgG control treated allergic animals. The reduction of food induced anaphylaxis was accompanied by a significant reduction in gut leak. The mesenteric lymph node cells were analyzed by flow cytometry and showed a decrease in the number of type 2 innate lymphoid cells in mice injected with α-SCF248. Morphometric enumeration of esterase+ mast cells demonstrated a significant reduction throughout the small intestine. Using a more chronic model of persistent food-induced anaphylaxis, short term therapeutic treatment with α-SCF248 during established disease effectively blocked food induced anaphylaxis. Together, these data suggest that therapeutically blocking SCF248 in food allergic animals can reduce the severity of food allergy by reducing mast cell mediated disease activation.
Keywords: anaphylaxis; food allergy; innate lymphoid cell; mast cell; stem cell factor.
Copyright © 2021 Ptaschinski, Rasky, Fonseca and Lukacs.
Conflict of interest statement
NL was a co-founder of Opsidio, LLC, which is developing anti-SCF248 for commercial use. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Eppikajutsuto Protects against Food Allergy Induced by Ovalbumin in a Murine Model.Int Arch Allergy Immunol. 2017;173(2):71-83. doi: 10.1159/000472715. Epub 2017 Jun 3. Int Arch Allergy Immunol. 2017. PMID: 28578324
-
Curcumin Ingestion Inhibits Mastocytosis and Suppresses Intestinal Anaphylaxis in a Murine Model of Food Allergy.PLoS One. 2015 Jul 6;10(7):e0132467. doi: 10.1371/journal.pone.0132467. eCollection 2015. PLoS One. 2015. PMID: 26147007 Free PMC article.
-
Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis.J Allergy Clin Immunol. 2013 Feb;131(2):451-60.e1-6. doi: 10.1016/j.jaci.2012.11.032. J Allergy Clin Immunol. 2013. PMID: 23374269 Free PMC article.
-
IL-9-producing cells in the development of IgE-mediated food allergy.Semin Immunopathol. 2017 Jan;39(1):69-77. doi: 10.1007/s00281-016-0605-x. Epub 2016 Dec 1. Semin Immunopathol. 2017. PMID: 27909880 Free PMC article. Review.
-
IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy.Front Immunol. 2020 Dec 11;11:603050. doi: 10.3389/fimmu.2020.603050. eCollection 2020. Front Immunol. 2020. PMID: 33362785 Free PMC article. Review.
Cited by
-
Human Lung Mast Cells: Therapeutic Implications in Asthma.Int J Mol Sci. 2022 Nov 21;23(22):14466. doi: 10.3390/ijms232214466. Int J Mol Sci. 2022. PMID: 36430941 Free PMC article. Review.
-
Inhibition of Soluble Stem Cell Factor Promotes Intestinal Mucosal Repair.Inflamm Bowel Dis. 2023 Jul 5;29(7):1133-1144. doi: 10.1093/ibd/izad003. Inflamm Bowel Dis. 2023. PMID: 36688460 Free PMC article.
-
Research Advances in Mast Cell Biology and Their Translation Into Novel Therapies for Anaphylaxis.J Allergy Clin Immunol Pract. 2023 Jul;11(7):2032-2042. doi: 10.1016/j.jaip.2023.03.015. Epub 2023 Mar 21. J Allergy Clin Immunol Pract. 2023. PMID: 36958519 Free PMC article. Review.
-
KIT as a master regulator of the mast cell lineage.J Allergy Clin Immunol. 2022 Jun;149(6):1845-1854. doi: 10.1016/j.jaci.2022.04.012. Epub 2022 Apr 22. J Allergy Clin Immunol. 2022. PMID: 35469840 Free PMC article. Review.
-
New Biomarkers in Anaphylaxis (Beyond Tryptase).Curr Treat Options Allergy. 2022;9(4):303-322. doi: 10.1007/s40521-022-00326-1. Epub 2022 Nov 28. Curr Treat Options Allergy. 2022. PMID: 36467524 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

