Bioinformatic Prediction and Production of Four Recombinant Proteins from Different Developmental Stages of Trichinella spiralis and Testing of Their Diagnostic Sensitivity in Mice
- PMID: 33786054
- PMCID: PMC7988681
- DOI: 10.18502/ijpa.v16i1.5531
Bioinformatic Prediction and Production of Four Recombinant Proteins from Different Developmental Stages of Trichinella spiralis and Testing of Their Diagnostic Sensitivity in Mice
Abstract
Background: Trichinellosis is a serious food-borne parasitic zoonosis, thus finding high quality antigens is the key to serodiagnosis of trichinosis. This article reports the characterization and sensitivity of four recombinant proteins expressed by four genes (Wn10, Zh68, T668, and Wm5) from different developmental stages of Trichinella spiralis for the diagnosis of trichinellosis in mice.
Methods: This study was conducted in Jilin University and National Institute of Parasitic Diseases of Chinese Center for Disease Control and Prevention in 2017-2018. The structures and functions of the proteins encoded by four genes were predicted by bioinformatics analysis. The four genes were cloned and expressed, and the recombinant proteins were purified. Anti-Trichinella IgM and IgG antibodies in the sera of mice infected with T. spiralis from 1-45 d post-infection (dpi) were evaluated by ELISA.
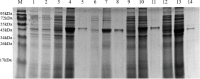
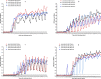
Results: The optimal antigen epitopes of four proteins (P1, P2, P3, and P4) encoded by the four genes from T- and B-cells were predicted, and four purified recombinant proteins (r-P1, r-P2, r-P3, and r-P4) were successfully produced. For IgM, the antibody levels detected by the four recombinant antigens were approximately equal to the cut-off value. Anti-Trichinella IgG antibodies were first detected by r-P1 at 8 dpi, followed by r-P2, r-P3, and r-P4 at 10 dpi, 14 dpi, and 16 dpi, respectively, and the antibody levels remained high until 45 dpi.
Conclusion: The recombinant antigens r-P1, r-P2, r-P3, and r-P4 could be antigens that react with antibodies, they showed high sensitivity in the detection of anti-Trichinella IgG antibodies in mice. Among these proteins, r-P1 may be a candidate antigen for the detection of anti-Trichinella IgG antibodies in the early infection phase and exhibited the best sensitivity among the antigens.
Keywords: Bioinformatics analysis; Diagnostic characteristics; Genes; Recombinant protein; Trichinella spiralis.
Copyright © 2021 Zhai et al. Published by Tehran University of Medical Sciences.
Conflict of interest statement
Conflict of interest We have no conflict of interest related to this study.
Figures







Similar articles
-
[The usefulness of ELISA test for early serological detection of Trichinella spp. infection in pigs].Wiad Parazytol. 2007;53(2):149-51. Wiad Parazytol. 2007. PMID: 17912813 Polish.
-
The Use of Recombinant 31 kDa Antigens of Trichinella spiralis for Serodiagnosis of Experimental Trichinellosis.Iran J Parasitol. 2015 Apr-Jun;10(2):230-7. Iran J Parasitol. 2015. PMID: 26246820 Free PMC article.
-
Kinetics Evaluation of IgM and IgG Levels in the Mice Infected with Trichinella spiralis Experimentally Using ES Antigens from Different Developmental Stages of the Parasite.Iran J Parasitol. 2019 Apr-Jun;14(2):223-230. Iran J Parasitol. 2019. PMID: 31543910 Free PMC article.
-
Serodiagnosis of trichinellosis by ELISA using recombinant nudix hydrolase of Trichinella spiralis.Trop Biomed. 2015 Dec 1;32(4):669-675. Trop Biomed. 2015. PMID: 33557457
-
[The influence of the procedure of excretory-secretory L1 trichinella spiralis antigen preparation on the efficiency of an ELISA test in pigs].Wiad Parazytol. 2006;52(3):219-30. Wiad Parazytol. 2006. PMID: 17432246 Polish.
References
-
- Feidas H, Kouam MK, Kantzoura V.Global geographic distribution of Trichinella species and genotypes. Infect Genet Evol. 2014; 26:255–266. - PubMed
-
- Bruschi F. Trichinellosis in developing countries: is it neglected? J Infect Dev Ctries. 2012; 6(3):216–222. - PubMed
-
- Rostami A, Gamble HR, Dupouy-Camet J, et al. Meat sources of infection for outbreaks of human trichinellosis. Food Microbiol. 2017; 64:65–71. - PubMed
-
- Murrell KD. The dynamics of Trichinella spiralis epidemiology: out to pasture? Vet Parasitol. 2016; 231:92–96. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
