A novel scoring system to estimate chemotherapy-induced myocardial toxicity: Risk assessment prior to non-anthracycline chemotherapy regimens
- PMID: 33786364
- PMCID: PMC7988329
- DOI: 10.1016/j.ijcha.2021.100751
A novel scoring system to estimate chemotherapy-induced myocardial toxicity: Risk assessment prior to non-anthracycline chemotherapy regimens
Abstract
Background: Myocardial toxicity is a common side effect of chemotherapy and is associated with adverse outcomes in cancer patients. Sufficient prediction of chemotherapy-induced myocardiotoxicity (CIMC) is desirable. Therefore, we sought to develop a feasible scoring system to predict CIMC in cancer patients undergoing non-anthracycline chemotherapy.
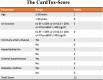
Methods: We determined a scoring system, the "Cardiotoxicitiy Score" (the CardTox-Score), by multivariable regression of the parameters considered relevant to the development of CIMC, based on previously published data and current guidelines. Variables of the risk model consist of clinical (age, presence of cardiovascular risk conditionsconditions), blood tests (NT-proBNP), and echocardiographic parameters (left ventricular (LV) ejection fraction, LV strain analysis). The CardTox-Score was examined in an internal validation cohort by use of ROC and regression analysis.
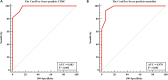
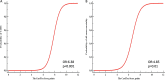
Results: We prospectively investigated 225 patients (58.21 ± 6.3 years, 52.8% female) who received non-anthracycline myocardiotoxic anticancer agent as a derivation cohort. All patients underwent echocardiography before, during and after anticancer therapy. The mean follow-up duration was 25 ± 4 months. We found the CardTox-Score (>6 points) to be a strong independent predictor (AUC: 0.983, OR: 6.38, 95% CI: 1.6 2.8, p < 0.001) for the development of CIMC with high sensitivity (100%) and specificity (84.2%) in the validation cohort (n = 30, 59.2 ± 6.5 years, 57% female). Moreover, the CardTox-Score appropriately predicted all-cause mortality with high specificity (93.7%) and sensitivity (92.9%) as well (OR: 4.85, AUC: 0.978, p = 0.01).
Conclusion: The CardTox-Score offers a promising, feasible, and easy-to-handle scoring system for predicting CIMC in cancer patients undergoing non-anthracycline regimes, independent from the type of cancer.
Keywords: AUC, Area under the curve; CI, Confidence interval; CK-MB, Creatine kinase isoenzyme MB; Cardiomyopathy; Cardiotoxicity; Chemotherapy; FU, Follow-up; LDL, Low-density lipoprotein; LV-EF, Left-ventricular ejection fraction; LV-GLS, Left-ventricular global longitudinal strain; NT-proBNP, N terminal pro-brain natriuretic peptide; OR, Odds ratio; ROC, Receiver operating characteristic; Risk assessment; Strain analysis.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Cardinale D., Ciceri F., Latini R., Franzosi M.G., Sandri M.T., Civelli M. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the international cardiooncology society-one trial. Eur. J. Cancer. 2018;94:126–137. doi: 10.1016/j.ejca.2018.02.005. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

