COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study
- PMID: 33786454
- PMCID: PMC7993930
- DOI: 10.1016/S2665-9913(21)00059-X
COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study
Abstract
Background: Various observations have suggested that the course of COVID-19 might be less favourable in patients with inflammatory rheumatic and musculoskeletal diseases receiving rituximab compared with those not receiving rituximab. We aimed to investigate whether treatment with rituximab is associated with severe COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases.
Methods: In this cohort study, we analysed data from the French RMD COVID-19 cohort, which included patients aged 18 years or older with inflammatory rheumatic and musculoskeletal diseases and highly suspected or confirmed COVID-19. The primary endpoint was the severity of COVID-19 in patients treated with rituximab (rituximab group) compared with patients who did not receive rituximab (no rituximab group). Severe disease was defined as that requiring admission to an intensive care unit or leading to death. Secondary objectives were to analyse deaths and duration of hospital stay. The inverse probability of treatment weighting propensity score method was used to adjust for potential confounding factors (age, sex, arterial hypertension, diabetes, smoking status, body-mass index, interstitial lung disease, cardiovascular diseases, cancer, corticosteroid use, chronic renal failure, and the underlying disease [rheumatoid arthritis vs others]). Odds ratios and hazard ratios and their 95% CIs were calculated as effect size, by dividing the two population mean differences by their SD. This study is registered with ClinicalTrials.gov, NCT04353609.
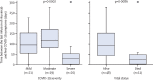
Findings: Between April 15, 2020, and Nov 20, 2020, data were collected for 1090 patients (mean age 55·2 years [SD 16·4]); 734 (67%) were female and 356 (33%) were male. Of the 1090 patients, 137 (13%) developed severe COVID-19 and 89 (8%) died. After adjusting for potential confounding factors, severe disease was observed more frequently (effect size 3·26, 95% CI 1·66-6·40, p=0·0006) and the duration of hospital stay was markedly longer (0·62, 0·46-0·85, p=0·0024) in the 63 patients in the rituximab group than in the 1027 patients in the no rituximab group. 13 (21%) of 63 patients in the rituximab group died compared with 76 (7%) of 1027 patients in the no rituximab group, but the adjusted risk of death was not significantly increased in the rituximab group (effect size 1·32, 95% CI 0·55-3·19, p=0·53).
Interpretation: Rituximab therapy is associated with more severe COVID-19. Rituximab will have to be prescribed with particular caution in patients with inflammatory rheumatic and musculoskeletal diseases.
Funding: None.
© 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare no competing interests.
Figures
References
-
- Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–858. - PubMed
-
- Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 3. Arthritis Rheumatol. 2021;73:e1–12. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

