Vedolizumab and Anti-Tumour Necrosis Factor α Real-World Outcomes in Biologic-Naïve Inflammatory Bowel Disease Patients: Results from the EVOLVE Study
- PMID: 33786600
- PMCID: PMC8495488
- DOI: 10.1093/ecco-jcc/jjab058
Vedolizumab and Anti-Tumour Necrosis Factor α Real-World Outcomes in Biologic-Naïve Inflammatory Bowel Disease Patients: Results from the EVOLVE Study
Abstract
Background and aims: This study aimed to compare real-world clinical effectiveness and safety of vedolizumab, an α4β7-integrin inhibitor, and anti-tumour necrosis factor-α [anti-TNFα] agents in biologic-naïve ulcerative colitis [UC] and Crohn's disease [CD] patients.
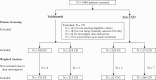
Methods: This was a 24-month retrospective medical chart study in adult UC and CD patients treated with vedolizumab or anti-TNFα in Canada, Greece and the USA. Inverse probability weighting was used to account for differences between groups. Primary outcomes were cumulative rates of clinical effectiveness [clinical response, clinical remission, mucosal healing] and incidence rates of serious adverse events [SAEs] and serious infections [SIs]. Secondary outcomes included cumulative rates of treatment persistence [patients who did not discontinue index treatment during follow-up] and dose escalation and incidence rates of disease exacerbations and disease-related surgeries. Adjusted analyses were performed using inverse probability weighting.
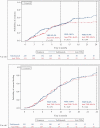
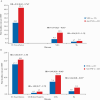
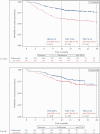
Results: A total of 1095 patients [604 UC, 491 CD] were included. By 24 months, rates of clinical effectiveness were similar between groups, but incidence rates of SAEs (hazard ratio [HR] = 0.42 [0.28-0.62]) and SIs (HR = 0.40 [0.19-0.85]) were significantly lower in vedolizumab vs anti-TNFα patients. Rates of treatment persistence [p < 0.01] by 24 months were higher in vedolizumab patients with UC. Incidence rates of disease exacerbations were lower in vedolizumab patients with UC (HR = 0.58 [0.45-0.76]). Other outcomes did not significantly differ between groups.
Conclusion: In this real-world setting, first-line biologic therapy in biologic-naïve patients with UC and CD demonstrated that vedolizumab and anti-TNFα treatments were equally effective at controlling disease symptoms, but vedolizumab has a more favourable safety profile.
Keywords: Vedolizumab; biologic-naïve; real-world effectiveness.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Figures

References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. - PubMed
-
- Ardizzone S, Bianchi Porro G. Biologic therapy for inflammatory bowel disease. Drugs 2005;65:2253–86. - PubMed
-
- Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis 2016;10:1437–44. - PubMed
-
- Feagan BG, Rubin DT, Danese S, et al. . Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol 2017;15:229–39.e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

