Magnetically Driven Micro and Nanorobots
- PMID: 33787235
- PMCID: PMC8154323
- DOI: 10.1021/acs.chemrev.0c01234
Magnetically Driven Micro and Nanorobots
Abstract
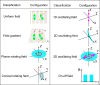
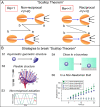
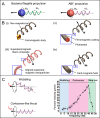
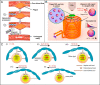
Manipulation and navigation of micro and nanoswimmers in different fluid environments can be achieved by chemicals, external fields, or even motile cells. Many researchers have selected magnetic fields as the active external actuation source based on the advantageous features of this actuation strategy such as remote and spatiotemporal control, fuel-free, high degree of reconfigurability, programmability, recyclability, and versatility. This review introduces fundamental concepts and advantages of magnetic micro/nanorobots (termed here as "MagRobots") as well as basic knowledge of magnetic fields and magnetic materials, setups for magnetic manipulation, magnetic field configurations, and symmetry-breaking strategies for effective movement. These concepts are discussed to describe the interactions between micro/nanorobots and magnetic fields. Actuation mechanisms of flagella-inspired MagRobots (i.e., corkscrew-like motion and traveling-wave locomotion/ciliary stroke motion) and surface walkers (i.e., surface-assisted motion), applications of magnetic fields in other propulsion approaches, and magnetic stimulation of micro/nanorobots beyond motion are provided followed by fabrication techniques for (quasi-)spherical, helical, flexible, wire-like, and biohybrid MagRobots. Applications of MagRobots in targeted drug/gene delivery, cell manipulation, minimally invasive surgery, biopsy, biofilm disruption/eradication, imaging-guided delivery/therapy/surgery, pollution removal for environmental remediation, and (bio)sensing are also reviewed. Finally, current challenges and future perspectives for the development of magnetically powered miniaturized motors are discussed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures














References
-
- Yan X.; Zhou Q.; Yu J.; Xu T.; Deng Y.; Tang T.; Feng Q.; Bian L.; Zhang Y.; Ferreira A.; et al. Magnetite Nanostructured Porous Hollow Helical Microswimmers for Targeted Delivery. Adv. Funct. Mater. 2015, 25, 5333–5342. 10.1002/adfm.201502248. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

