An expanding manifold in transmodal regions characterizes adolescent reconfiguration of structural connectome organization
- PMID: 33787489
- PMCID: PMC8087442
- DOI: 10.7554/eLife.64694
An expanding manifold in transmodal regions characterizes adolescent reconfiguration of structural connectome organization
Abstract
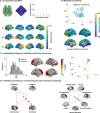
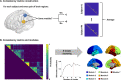
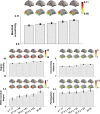
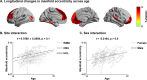
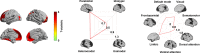
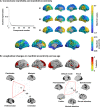
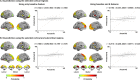
Adolescence is a critical time for the continued maturation of brain networks. Here, we assessed structural connectome development in a large longitudinal sample ranging from childhood to young adulthood. By projecting high-dimensional connectomes into compact manifold spaces, we identified a marked expansion of structural connectomes, with strongest effects in transmodal regions during adolescence. Findings reflected increased within-module connectivity together with increased segregation, indicating increasing differentiation of higher-order association networks from the rest of the brain. Projection of subcortico-cortical connectivity patterns into these manifolds showed parallel alterations in pathways centered on the caudate and thalamus. Connectome findings were contextualized via spatial transcriptome association analysis, highlighting genes enriched in cortex, thalamus, and striatum. Statistical learning of cortical and subcortical manifold features at baseline and their maturational change predicted measures of intelligence at follow-up. Our findings demonstrate that connectome manifold learning can bridge the conceptual and empirical gaps between macroscale network reconfigurations, microscale processes, and cognitive outcomes in adolescent development.
Keywords: adolescence; connectome; human; longitudinal; multi-scale; neurodevelopment; neuroimaging; neuroscience.
© 2021, Park et al.
Conflict of interest statement
BP, RB, CP, SL, RR, RV, BB No competing interests declared, EB ETB. serves on the scientific advisory board of Sosei Heptares and as a consultant for GlaxoSmithKline.
Figures











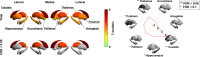



References
-
- Aglioti S. The role of the thalamus and basal ganglia in human cognition. Journal of Neurolinguistics. 1997;10:255–265. doi: 10.1016/S0911-6044(97)00020-1. - DOI
-
- Altmann A, Ryten M, Di NM, Ravizza T, Tolomeo D, Reynolds RH, Somani A, Bacigaluppi M, Iori V, Micotti E, Botia JA, Absil J, Alhusaini S, Alvim MKM, Auvinen P, Bargallo N, Bartolini E, Bender B, Bergo F, Bernardes T, Bernasconi A, Bernasconi N, Bernhardt BC, Blackmon K, Braga B, Caligiuri ME, Cavalo A, Carlson C, Carr S, Cavalleri G, Cendes F, Chen J, Chen S, Cherubini A, Concha L, David P, Delanty N, Depondt C, Devinsky O, Doherty CP, Domin M, Focke N, Foley S, Franca W, Gambardella A, Guerrini R, Hamandi K, Hibar DP, Isaev DY, Jackson G, Jahanshad N, Kalviainen R, Keller S, Kochunov P, Kotikalapudi R, Kowalczyk MA, Kuzniecky R, Kwan P, Labate A, Langer S, Lenge M, Liu M, Martin P, Mascalchi M, Meletti S, Morita ME, O’Brien TJ, Pariente JC, Richardson MP, Rodriguez-Cruces R, Rummel C, Saavalainen T, Semmelroch MK, Severino M, Striano P, Thesen T, Thomas RH, Tondelli M, Tortora D, Vaudano AE, Vivash L, von PF, Wagner J, Weber B, Wiest R, Yasuda CL, Zhang G, Zhang J, Group E-EW, Leu C, Avbersek A, Consortium E, Thom M, Whelan CD, Thompson P, McDonald C, Vezzani A, Sisodiya SM. A systems-level analysis highlights microglial activation as a modifying factor in common forms of human epilepsy. bioRxiv. 2018 doi: 10.1101/470518. - DOI - PMC - PubMed
-
- Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, Thiebaut de Schotten M. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Communications Biology. 2019;2:370. doi: 10.1038/s42003-019-0611-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

