Vitamin D supplementation and improvement of pneumonic children at a tertiary pediatric hospital in Egypt: A randomized controlled trial
- PMID: 33787584
- PMCID: PMC8021283
- DOI: 10.1097/MD.0000000000025011
Vitamin D supplementation and improvement of pneumonic children at a tertiary pediatric hospital in Egypt: A randomized controlled trial
Abstract
Background: Despite the well-recognized effect of vitamin D in metabolism and homeostasis, there is now growing interest in its probable association with pneumonia. This study aims to supply vitamin D3 (Cholecalciferol) (100,000 IU) to pneumonic children to minimize the duration of illness and improve their outcome.
Methods: A double-blinded, randomized, placebo-controlled trial was conducted in a Pediatric Cairo University affiliated hospital. An intervention arm (93 children) and a control arm (98 children), who had pneumonia with an insufficient or deficient level of vitamin D and whose parental permission was obtained, were enrolled in the trial. All children were treated with antibiotics according to WHO guidelines. Children were given a single injection of 1 mL of 100,000 IU of vitamin D3 or placebo. Clinical data were recorded every eight hours for all children. Outcomes were assessed 7 days after vitamin D injection.The primary outcome variable was the change in serum level of 25(OH)D, while the secondary outcomes were the medical state of the assigned cases (improvement or death) and duration between enrollment and hospital discharge for improved cases.
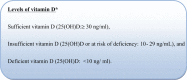
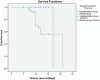
Results: In the supplementation group, the percentage of patients who suffered either deficient (38.7%) or insufficient levels (61.3%) of 25 (OH)D at day one had significantly decreased in the seventh day to (11.8%) and (52.7%), respectively. Kaplan--Meier plots highlighted that the median time to recover of the placebo group was significantly longer than that of the supplementation group (Log Rank P value < .001).
Conclusion: VDD was detected in pediatric critical care children. In pneumonic children with high VDD, it is illustrated that Vitamin D supplementation is accompanied by lowered mortality risk and pSOFA scores, reduced time to recover, and improved PaO2/FiO2.
Trial registration: Trial Identifier number: NCT04244474. Registered on 27 January 2020- Retrospectively registered at ClinicalTrials.gov https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S0009JXO&selectaction=Edit&uid=U0004UO8&ts=152&cx=9cceq6.
Copyright © 2021 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
A phase II dose evaluation pilot feasibility randomized controlled trial of cholecalciferol in critically ill children with vitamin D deficiency (VITdAL-PICU study).BMC Pediatr. 2023 Aug 14;23(1):397. doi: 10.1186/s12887-023-04205-9. BMC Pediatr. 2023. PMID: 37580663 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled trial of vitamin D supplementation with or without calcium in community-dwelling vitamin D deficient subjects.BMC Musculoskelet Disord. 2022 May 3;23(1):415. doi: 10.1186/s12891-022-05364-z. BMC Musculoskelet Disord. 2022. PMID: 35505326 Free PMC article. Clinical Trial.
-
Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial.JAMA. 2020 Aug 25;324(8):752-760. doi: 10.1001/jama.2020.12384. JAMA. 2020. PMID: 32840597 Free PMC article. Clinical Trial.
-
Vitamin D supplementation for sickle cell disease.Cochrane Database Syst Rev. 2020 May 28;5(5):CD010858. doi: 10.1002/14651858.CD010858.pub3. Cochrane Database Syst Rev. 2020. PMID: 32462740 Free PMC article.
-
Vitamin D deficiency and acute lower respiratory infections in children younger than 5 years: identification and treatment.J Pediatr Health Care. 2014 Nov-Dec;28(6):572-82; quiz 583-4. doi: 10.1016/j.pedhc.2014.08.013. Epub 2014 Oct 18. J Pediatr Health Care. 2014. PMID: 25441970 Free PMC article. Review.
Cited by
-
Vitamin D as an adjunct to antibiotics for the treatment of acute childhood pneumonia.Cochrane Database Syst Rev. 2023 Jan 12;1(1):CD011597. doi: 10.1002/14651858.CD011597.pub3. Cochrane Database Syst Rev. 2023. PMID: 36633175 Free PMC article.
-
Rapid normalization of vitamin D deficiency in PICU (VITdALIZE-KIDS): study protocol for a phase III, multicenter randomized controlled trial.Trials. 2024 Sep 19;25(1):619. doi: 10.1186/s13063-024-08461-7. Trials. 2024. PMID: 39300483 Free PMC article.
-
Immunomodulatory Effects of Vitamin D in Respiratory Tract Infections and COVID-19 in Children.Nutrients. 2023 Aug 2;15(15):3430. doi: 10.3390/nu15153430. Nutrients. 2023. PMID: 37571367 Free PMC article. Review.
-
Dietary Supplements among Children Ages 0-3 Years in Poland-Are They Necessary?Foods. 2022 Dec 21;12(1):16. doi: 10.3390/foods12010016. Foods. 2022. PMID: 36613232 Free PMC article.
-
The effects of vitamin D on all-cause mortality in different diseases: an evidence-map and umbrella review of 116 randomized controlled trials.Front Nutr. 2023 Jun 22;10:1132528. doi: 10.3389/fnut.2023.1132528. eCollection 2023. Front Nutr. 2023. PMID: 37426183 Free PMC article.
References
-
- Elsevier, Kliegman RM, Geme JSt. Nelson Textbook of Pediatrics. 21th ed.2019;2088-94.
-
- UNICEF. Levels & Trends in Child Mortality. Estimates developed by the UN Inter-agency Group for Child Mortality Estimation. Report 2019. Available at: https://childmortality.org/wpcontent/uploads/2019/10/UN-IGME-Child-Morta.... Accessed March, 2019.
-
- Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008;88:491S–9S. - PubMed
-
- Underwood MA, Bevins CL. Defensin-barbed innate immunity: clinical associations in the pediatric population. Pediatrics 2010;125:1237–47. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical