Evaluation of the RSNA and CORADS classifications for COVID-19 on chest computed tomography in the Brazilian population
- PMID: 33787655
- PMCID: PMC7979034
- DOI: 10.6061/clinics/2021/e2476
Evaluation of the RSNA and CORADS classifications for COVID-19 on chest computed tomography in the Brazilian population
Abstract
Objective: To determine the correlation between the two tomographic classifications for coronavirus disease (COVID-19), COVID-19 Reporting and Data System (CORADS) and Radiological Society of North America Expert Consensus Statement on Reporting Chest Computed Tomography (CT) Findings Related to COVID-19 (RSNA), in the Brazilian population and to assess the agreement between reviewers with different experience levels.
Methods: Chest CT images of patients with reverse transcriptase-polymerase chain reaction (RT-PCR)-positive COVID-19 were categorized according to the CORADS and RSNA classifications by radiologists with different levels of experience and who were initially unaware of the RT-PCR results. The inter- and intra-observer concordances for each of the classifications were calculated, as were the concordances between classifications.
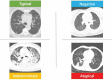
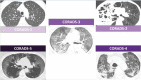
Results: A total of 100 patients were included in this study. The RSNA classification showed an almost perfect inter-observer agreement between reviewers with similar experience levels, with a kappa coefficient of 0.892 (95% confidence interval [CI], 0.788-0.995). CORADS showed substantial agreement among reviewers with similar experience levels, with a kappa coefficient of 0.642 (95% CI, 0.491-0.793). There was inter-observer variation when comparing less experienced reviewers with more experienced reviewers, with the highest kappa coefficient of 0.396 (95% CI, 0.255-0.588). There was a significant correlation between both classifications, with a Kendall coefficient of 0.899 (p<0.001) and substantial intra-observer agreement for both classifications.
Conclusion: The RSNA and CORADS classifications showed excellent inter-observer agreement for reviewers with the same level of experience, although the agreement between less experience reviewers and the reviewer with the most experience was only reasonable. Combined analysis of both classifications with the first RT-PCR results did not reveal any false-negative results for detecting COVID-19 in patients.
Conflict of interest statement
No potential conflict of interest was reported.
Figures
Similar articles
-
Radiological Society of North America (RSNA) Expert Consensus Statement Related to Chest CT Findings in COVID-19 Versus CO-RADS: Comparison of Reporting System Performance Among Chest Radiologists and End-User Preference.Can Assoc Radiol J. 2021 Nov;72(4):806-813. doi: 10.1177/0846537120968919. Epub 2020 Nov 3. Can Assoc Radiol J. 2021. PMID: 33138634
-
RSNA Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19: Interobserver Agreement Between Chest Radiologists.Can Assoc Radiol J. 2021 Feb;72(1):159-166. doi: 10.1177/0846537120938328. Epub 2020 Jul 2. Can Assoc Radiol J. 2021. PMID: 32615802 Free PMC article.
-
Interobserver and Intraobserver Variability in the CT Assessment of COVID-19 Based on RSNA Consensus Classification Categories.Acad Radiol. 2020 Nov;27(11):1499-1506. doi: 10.1016/j.acra.2020.08.038. Epub 2020 Sep 15. Acad Radiol. 2020. PMID: 32948442 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD013639. doi: 10.1002/14651858.CD013639.pub4. PMID: 33242342 Updated.
Cited by
-
Inter-observer Variability in the Analysis of CO-RADS Classification for COVID-19 Patients.Trop Med Infect Dis. 2023 Dec 17;8(12):523. doi: 10.3390/tropicalmed8120523. Trop Med Infect Dis. 2023. PMID: 38133455 Free PMC article.
-
Analysis of Leukocyte Subpopulations by Flow Cytometry during Hospitalization Depending on the Severity of COVID-19 Course.Biomedicines. 2023 Oct 8;11(10):2728. doi: 10.3390/biomedicines11102728. Biomedicines. 2023. PMID: 37893102 Free PMC article.
-
Differentiating Leukostasis From COVID-19 Pneumonia: Clinical and Radiological Perspectives for the Right Decision-Making.Cureus. 2024 Mar 22;16(3):e56708. doi: 10.7759/cureus.56708. eCollection 2024 Mar. Cureus. 2024. PMID: 38646395 Free PMC article.
-
Diagnostic Efficacy of Chest Computed Tomography with a Dual-Reviewer Approach in Patients Diagnosed with Pneumonia Secondary to Severe Acute Respiratory Syndrome Coronavirus 2.Tomography. 2023 Aug 24;9(5):1617-1628. doi: 10.3390/tomography9050129. Tomography. 2023. PMID: 37736982 Free PMC article.
-
Comparison of Initial CT Findings and CO-RADS Stage in COVID-19 Patients with PCR, Inflammation and Coagulation Parameters in Diagnostic and Prognostic Perspectives.J Belg Soc Radiol. 2022 Jul 8;106(1):67. doi: 10.5334/jbsr.2714. eCollection 2022. J Belg Soc Radiol. 2022. PMID: 35859920 Free PMC article.
References
-
- Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological Society of North America Expert Consensus Document on Reporting Chest CT Findings Related to COVID-19: Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothorac Imaging. 2020;2(2):e200152. doi: 10.1148/ryct.2020200152. - DOI - PMC - PubMed
-
- Miranda Magalhães Santos JM, Paula Alves Fonseca A, Pinheiro Zarattini Anastacio E, Formagio Minenelli F, Furtado de Albuquerque Cavalcanti C, Borges da Silva Teles G. Initial Results of the Use of a Standardized Diagnostic Criteria for Chest Computed Tomography Findings in Coronavirus Disease 2019. J Comput Assist Tomogr. 2020;44(5):647–51. doi: 10.1097/RCT.0000000000001054. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

