Tumor Cellularity and Infiltrating Lymphocytes as a Survival Surrogate in HER2-Positive Breast Cancer
- PMID: 33787900
- PMCID: PMC8902438
- DOI: 10.1093/jnci/djab057
Tumor Cellularity and Infiltrating Lymphocytes as a Survival Surrogate in HER2-Positive Breast Cancer
Abstract
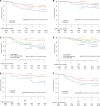
In early-stage HER2-positive breast cancer, biomarkers that guide deescalation and/or escalation of systemic therapy are needed. CelTIL score is a novel, combined biomarker based on stromal tumor-infiltrating lymphocytes and tumor cellularity and is determined in tumor biopsies at week 2 of anti-HER2 therapy only. We evaluated the prognostic value of CelTIL in 196 patients with early-stage HER2-positive disease treated with standard trastuzumab-based chemotherapy in the NeoALTTO phase III trial. Using a prespecified CelTIL cutoff, a better 5-year event-free survival and overall survival was observed between CelTIL-high and CelTIL-low score with a 76.4% (95% confidence interval [CI] = 68.0% to 85.0%) vs 59.7% (95% CI = 50.0% to 72.0%) (hazard ratio = 0.40, 95% CI = 0.17 to 0.94) and 86.4% (95% CI = 80.0% to 94.0%) vs 73.5% (95% CI = 64.0% to 84.0%) (hazard ratio = 0.43, 95% CI = 0.20 to 0.92), respectively. Statistical significance was maintained after adjusting for baseline tumor-infiltrating lymphocytes, hormone receptor status, pretreatment tumor size and nodal status, type of surgery, treatment arm, and pathological complete response. Further studies to support CelTIL as an early readout biomarker to help deescalate or escalate systemic therapy in HER2-positive breast cancer seem warranted.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
On the Quest of Risk Stratification in HER2-Positive Breast Cancer.J Natl Cancer Inst. 2022 Mar 8;114(3):345-346. doi: 10.1093/jnci/djab061. J Natl Cancer Inst. 2022. PMID: 33787921 Free PMC article. No abstract available.
References
-
- Burstein HJ, Curigliano G, Loibl S, et al. ; Members of the St. Gallen International Consensus Panel on the Primary Therapy of Early Breast Cancer 2019. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541–1557. - PubMed
-
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688–1700. - PubMed
-
- von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

