Association Between Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis
- PMID: 33787911
- PMCID: PMC8013817
- DOI: 10.1001/jamanetworkopen.2021.3594
Association Between Renin-Angiotensin-Aldosterone System Inhibitors and Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis
Abstract
Importance: The chronic receipt of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) has been assumed to exacerbate complications associated with COVID-19 and produce worse clinical outcomes.
Objective: To conduct an updated and comprehensive systematic review and meta-analysis comparing mortality and severe adverse events (AEs) associated with receipt vs nonreceipt of ACEIs or ARBs among patients with COVID-19.
Data sources: PubMed and Embase databases were systematically searched from December 31, 2019, until September 1, 2020.
Study selection: The meta-analysis included any study design, with the exception of narrative reviews or opinion-based articles, in which COVID-19 was diagnosed through laboratory or radiological test results and in which clinical outcomes (unadjusted or adjusted) associated with COVID-19 were assessed among adult patients (≥18 years) receiving ACEIs or ARBs.
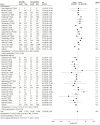
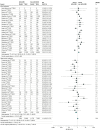
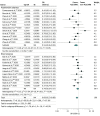
Data extraction and synthesis: Three authors independently extracted data on mortality and severe AEs associated with COVID-19. Severe AEs were defined as intensive care unit admission or the need for assisted ventilation. For each outcome, a random-effects model was used to compare the odds ratio (OR) between patients receiving ACEIs or ARBs vs those not receiving ACEIs or ARBs.
Main outcomes and measures: Unadjusted and adjusted ORs for mortality and severe AEs associated with COVID-19.
Results: A total of 1788 records from the PubMed and Embase databases were identified; after removal of duplicates, 1664 records were screened, and 71 articles underwent full-text evaluation. Clinical data were pooled from 52 eligible studies (40 cohort studies, 6 case series, 4 case-control studies, 1 randomized clinical trial, and 1 cross-sectional study) enrolling 101 949 total patients, of whom 26 545 (26.0%) were receiving ACEIs or ARBs. When adjusted for covariates, significant reductions in the risk of death (adjusted OR [aOR], 0.57; 95% CI, 0.43-0.76; P < .001) and severe AEs (aOR, 0.68; 95% CI, 0.53-0.88; P < .001) were found. Unadjusted and adjusted analyses of a subgroup of patients with hypertension indicated decreases in the risk of death (unadjusted OR, 0.66 [95% CI, 0.49-0.91]; P = .01; aOR, 0.51 [95% CI, 0.32-0.84]; P = .008) and severe AEs (unadjusted OR, 0.70 [95% CI, 0.54-0.91]; P = .007; aOR, 0.55 [95% CI, 0.36-0.85]; P = .007).
Conclusions and relevance: In this systematic review and meta-analysis, receipt of ACEIs or ARBs was not associated with a higher risk of multivariable-adjusted mortality and severe AEs among patients with COVID-19 who had either hypertension or multiple comorbidities, supporting the recommendations of medical societies. On the contrary, ACEIs and ARBs may be associated with protective benefits, particularly among patients with hypertension. Future randomized clinical trials are warranted to establish causality.
Conflict of interest statement
Figures

Similar articles
-
A systematic review and meta-analysis of the use of renin-angiotensin system drugs and COVID-19 clinical outcomes: What is the evidence so far?Pharmacol Res Perspect. 2020 Dec;8(6):e00666. doi: 10.1002/prp2.666. Pharmacol Res Perspect. 2020. PMID: 33084232 Free PMC article.
-
A Retrospective Study from 2 Centers in China on the Effects of Continued Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Patients with Hypertension and COVID-19.Med Sci Monit. 2020 Sep 24;26:e926651. doi: 10.12659/MSM.926651. Med Sci Monit. 2020. PMID: 32969367 Free PMC article.
-
Comparison of renin-angiotensin-aldosterone system inhibitors with other antihypertensives in association with coronavirus disease-19 clinical outcomes.BMC Infect Dis. 2021 Jun 5;21(1):527. doi: 10.1186/s12879-021-06088-6. BMC Infect Dis. 2021. PMID: 34090358 Free PMC article.
-
The divergent protective effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on clinical outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis.Ann Palliat Med. 2022 Apr;11(4):1253-1263. doi: 10.21037/apm-21-972. Epub 2021 Nov 4. Ann Palliat Med. 2022. PMID: 34775774
-
Estimation of Renin-Angiotensin-Aldosterone-System (RAAS)-Inhibitor effect on COVID-19 outcome: A Meta-analysis.J Infect. 2020 Aug;81(2):276-281. doi: 10.1016/j.jinf.2020.05.052. Epub 2020 May 28. J Infect. 2020. PMID: 32474043 Free PMC article.
Cited by
-
Kidney Injury in COVID-19: Epidemiology, Molecular Mechanisms and Potential Therapeutic Targets.Int J Mol Sci. 2022 Feb 17;23(4):2242. doi: 10.3390/ijms23042242. Int J Mol Sci. 2022. PMID: 35216358 Free PMC article. Review.
-
Attenuation of SARS-CoV-2 infection by losartan in human kidney organoids.iScience. 2022 Feb 18;25(2):103818. doi: 10.1016/j.isci.2022.103818. Epub 2022 Jan 28. iScience. 2022. PMID: 35106453 Free PMC article.
-
Angiotensin-Converting Enzyme (ACE) Inhibitors May Moderate COVID-19 Hyperinflammatory Response: An Observational Study with Deep Immunophenotyping.Health Data Sci. 2022;2022:0002. doi: 10.34133/hds.0002. Epub 2022 Dec 27. Health Data Sci. 2022. PMID: 36817759 Free PMC article.
-
Renin-angiotensin system blockers during the COVID-19 pandemic: an update for patients with hypertension and chronic kidney disease.Clin Kidney J. 2021 Dec 14;15(3):397-406. doi: 10.1093/ckj/sfab272. eCollection 2022 Mar. Clin Kidney J. 2021. PMID: 35198155 Free PMC article. Review.
-
Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and COVID-19-related outcomes: A patient-level analysis of the PCORnet blood pressure control lab.Am Heart J Plus. 2022 Jan;13:100112. doi: 10.1016/j.ahjo.2022.100112. Epub 2022 Mar 2. Am Heart J Plus. 2022. PMID: 35252907 Free PMC article.
References
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Updated February 13, 2021. Accessed January 18, 2021. https://covid19.who.int/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

