Results of a Dose-Finding Phase 1b Study of Subcutaneous Atezolizumab in Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer
- PMID: 33788415
- PMCID: PMC8518371
- DOI: 10.1002/cpdd.936
Results of a Dose-Finding Phase 1b Study of Subcutaneous Atezolizumab in Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer
Abstract
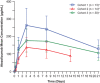
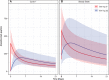
Intravenous (IV) atezolizumab is approved for non-small cell lung and other cancers. Subcutaneous (SC) atezolizumab coformulated with recombinant human hyaluronidase, a permeation enhancer for SC dispersion and absorption, is being developed to improve treatment options, reduce burden, and increase efficiency for patients and practitioners. IMscin001 (NCT03735121), a 2-part, open-label, global, multicenter, phase 1b/3 study, is evaluating the pharmacokinetics (PK), safety, and efficacy of SC atezolizumab. The part 1 (phase 1b) objective was determination of an SC atezolizumab dose yielding a serum trough concentration (Ctrough ) comparable with IV. Patients enrolled in 3 cohorts received SC atezolizumab 1800 mg (thigh) once (cohort 1), 1200 mg (thigh) every 2 weeks for 3 cycles (cohort 2), or 1800 mg (abdomen) every 3 weeks cycle 1, then cycles 2 and 3 (thigh) every 3 weeks (cohort 3). In subsequent cycles, IV atezolizumab 1200 mg every 3 weeks was administered until loss of clinical benefit. SC atezolizumab 1800 mg every 3 weeks and 1200 mg every 2 weeks provided similar Ctrough and area under the curve values in cycle 1 to the corresponding IV atezolizumab reference, was well tolerated, and exhibited a safety profile consistent with the established IV formulation. Exposure following SC injection in the abdomen was lower (20%, 28%, and 27% for Ctrough , maximum concentration, and area under the concentration-time curve from time 0 to day 21, respectively) than in the thigh. Part 1 SC and IV PK data were analyzed using a population PK modeling approach, followed by simulations. Part 2 (phase 3) will now be initiated to demonstrate that SC atezolizumab PK exposure is not lower than that of IV.
Keywords: NSCLC; atezolizumab; hyaluronanidase; pharmacokinetics; subcutaneous.
© 2021 The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
L.A.H.‐B. and E.R. are employees of and hold stock in F. Hoffmann‐La Roche. P.C. is an employee of Genentech/Roche and holds stock in Roche Holding Ltd. S.K. is an employee of ProUnlimited. V.M., P.C., and B.W. are employees of Genentech, Inc. E.P., M.M., and N.T. are employees of F. Hoffmann‐La Roche Ltd. M.M. is an employee of Certara and has received consulting fees from Genentech, Inc., in connection with this work. K.T. is an employee of Chugai Pharmaceutical Co., Ltd., and has received financial support from Genentech, Inc. All authors received support for third‐party writing assistance for this manuscript, provided by F. Hoffmann‐La Roche Ltd.
Figures
Comment in
-
Subcutaneous Atezolizumab: A Jab Without a Benefit.Clin Pharmacol Drug Dev. 2022 Jan;11(1):134-135. doi: 10.1002/cpdd.1061. Epub 2021 Dec 23. Clin Pharmacol Drug Dev. 2022. PMID: 34951144 No abstract available.
References
-
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837‐1846. - PubMed
-
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(7):924‐937. - PubMed
-
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288‐2301. - PubMed
-
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med. 2018;379(22):2108‐2121. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

