Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers
- PMID: 33788882
- PMCID: PMC8011749
- DOI: 10.1371/journal.pone.0248921
Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers
Abstract
Objectives: Determine the diagnostic accuracy of two antigen-detecting rapid diagnostic tests (Ag-RDT) for SARS-CoV-2 at the point of care and define individuals' characteristics providing best performance.
Methods: We performed a prospective, single-center, point of care validation of two Ag-RDT in comparison to RT-PCR on nasopharyngeal swabs.
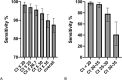
Results: Between October 9th and 23rd, 2020, 1064 participants were enrolled. The PanbioTM Covid-19 Ag Rapid Test device (Abbott) was validated in 535 participants, with 106 positive Ag-RDT results out of 124 positive RT-PCR individuals, yielding a sensitivity of 85.5% (95% CI: 78.0-91.2). Specificity was 100.0% (95% CI: 99.1-100) in 411 RT-PCR negative individuals. The Standard Q Ag-RDT (SD Biosensor, Roche) was validated in 529 participants, with 170 positive Ag-RDT results out of 191 positive RT-PCR individuals, yielding a sensitivity of 89.0% (95%CI: 83.7-93.1). One false positive result was obtained in 338 RT-PCR negative individuals, yielding a specificity of 99.7% (95%CI: 98.4-100). For individuals presenting with fever 1-5 days post symptom onset, combined Ag-RDT sensitivity was above 95%. Lower sensitivity of 88.2% was seen on the same day of symptom development (day 0).
Conclusions: We provide an independent validation of two widely available commercial Ag-RDTs, both meeting WHO criteria of ≥80% sensitivity and ≥97% specificity. Although less sensitive than RT-PCR, these assays could be beneficial due to their rapid results, ease of use, and independence from existing laboratory structures. Testing criteria focusing on patients with typical symptoms in their early symptomatic period onset could further increase diagnostic value.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Antigen-detection in the diagnosis of SARS-CoV-2 infection using rapid immunoassays [Internet]. 2020 [cited 2020 Oct 8]. Available from: https://www.who.int/publications-detail-redirect/antigen-detection-in-th...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

