Drought affects the rate and duration of organ growth but not inter-organ growth coordination
- PMID: 33788927
- PMCID: PMC8195526
- DOI: 10.1093/plphys/kiab155
Drought affects the rate and duration of organ growth but not inter-organ growth coordination
Abstract
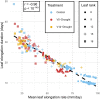
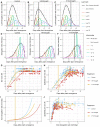
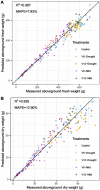
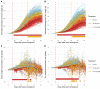
Drought at flowering and grain filling greatly reduces maize (Zea mays) yield. Climate change is causing earlier and longer-lasting periods of drought, which affect the growth of multiple maize organs throughout development. To study how long periods of water deficit impact the dynamic nature of growth, and to determine how these relate to reproductive drought, we employed a high-throughput phenotyping platform featuring precise irrigation, imaging systems, and image-based biomass estimations. Prolonged drought resulted in a reduction of growth rate of individual organs-though an extension of growth duration partially compensated for this-culminating in lower biomass and delayed flowering. However, long periods of drought did not affect the highly organized succession of maximal growth rates of the distinct organs, i.e. leaves, stems, and ears. Two drought treatments negatively affected distinct seed yield components: Prolonged drought mainly reduced the number of spikelets, and drought during the reproductive period increased the anthesis-silking interval. The identification of these divergent biomass and yield components, which were affected by the shift in duration and intensity of drought, will facilitate trait-specific breeding toward future climate-resilient crops.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Anami SE, Mgutu AJ, Taracha C, Coussens G, Karimi M, Hilson P, Van Lijsebettens M, Machuka J (2010) Somatic embryogenesis and plant regeneration of tropical maize genotypes. Plant Cell Tiss Organ Cult 102: 285–295
-
- Asaari MSM, Mertens S, Dhondt S, Inzé D, Wuyts N, Scheunders P (2019) Analysis of hyperspectral images for detection of drought stress and recovery in maize plants in a high-throughput phenotyping platform. Comput Electron Agric 162: 749–758
-
- Bechtold U, Penfold CA, Jenkins DJ, Legaie R, Moore JD, Lawson T, Matthews JSA, Vialet-Chabrand SRM, Baxter L, Subramaniam S, et al. (2016) Time-series transcriptomics reveals that AGAMOUS-LIKE22 affects primary metabolism and developmental processes in drought-stressed Arabidopsis. Plant Cell 28: 345–366 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

