Dynamic interplay between cell membrane tension and clathrin-mediated endocytosis
- PMID: 33788963
- PMCID: PMC8898183
- DOI: 10.1111/boc.202000110
Dynamic interplay between cell membrane tension and clathrin-mediated endocytosis
Abstract
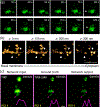
Deformability of the plasma membrane, the outermost surface of metazoan cells, allows cells to be dynamic, mobile and flexible. Factors that affect this deformability, such as tension on the membrane, can regulate a myriad of cellular functions, including membrane resealing, cell motility, polarisation, shape maintenance, membrane area control and endocytic vesicle trafficking. This review focuses on mechanoregulation of clathrin-mediated endocytosis (CME). We first delineate the origins of cell membrane tension and the factors that yield to its spatial and temporal fluctuations within cells. We then review the recent literature demonstrating that tension on the membrane is a fast-acting and reversible regulator of CME. Finally, we discuss tension-based regulation of endocytic clathrin coat formation during physiological processes.
Keywords: Cell migration/adhesion; Cellular imaging; Clathrin; Endocytosis/exocytosis; Membrane tension; Membrane trafficking.
© 2021 Société Française des Microscopies and Société de Biologie Cellulaire de France. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

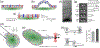


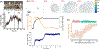
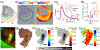

Similar articles
-
Membrane mechanics govern spatiotemporal heterogeneity of endocytic clathrin coat dynamics.Mol Biol Cell. 2017 Nov 15;28(24):3480-3488. doi: 10.1091/mbc.E17-05-0282. Epub 2017 Sep 13. Mol Biol Cell. 2017. PMID: 28904210 Free PMC article.
-
Design principles for robust vesiculation in clathrin-mediated endocytosis.Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1118-E1127. doi: 10.1073/pnas.1617705114. Epub 2017 Jan 26. Proc Natl Acad Sci U S A. 2017. PMID: 28126722 Free PMC article.
-
Minimal mesoscale model for protein-mediated vesiculation in clathrin-dependent endocytosis.PLoS Comput Biol. 2010 Sep 9;6(9):e1000926. doi: 10.1371/journal.pcbi.1000926. PLoS Comput Biol. 2010. PMID: 20838575 Free PMC article.
-
Mechanisms of clathrin-mediated endocytosis.Nat Rev Mol Cell Biol. 2018 May;19(5):313-326. doi: 10.1038/nrm.2017.132. Epub 2018 Feb 7. Nat Rev Mol Cell Biol. 2018. PMID: 29410531 Review.
-
Endocytosis: clathrin-mediated membrane budding.Curr Opin Cell Biol. 2007 Aug;19(4):417-25. doi: 10.1016/j.ceb.2007.05.003. Epub 2007 Jul 13. Curr Opin Cell Biol. 2007. PMID: 17631994 Review.
Cited by
-
Emerging roles and mechanisms of ERK pathway mechanosensing.Cell Mol Life Sci. 2023 Nov 10;80(12):355. doi: 10.1007/s00018-023-05007-z. Cell Mol Life Sci. 2023. PMID: 37947896 Free PMC article. Review.
-
How sticky? How tight? How hot? Imaging probes for fluid viscosity, membrane tension and temperature measurements at the cellular level.Int J Biochem Cell Biol. 2022 Dec;153:106329. doi: 10.1016/j.biocel.2022.106329. Epub 2022 Nov 3. Int J Biochem Cell Biol. 2022. PMID: 36336304 Free PMC article. Review.
-
Actin polymerization promotes invagination of flat clathrin-coated lattices in mammalian cells by pushing at lattice edges.Nat Commun. 2022 Oct 17;13(1):6127. doi: 10.1038/s41467-022-33852-2. Nat Commun. 2022. PMID: 36253374 Free PMC article.
-
Tensing Flipper: Photosensitized Manipulation of Membrane Tension, Lipid Phase Separation, and Raft Protein Sorting in Biological Membranes.J Am Chem Soc. 2024 Aug 28;146(34):24114-24124. doi: 10.1021/jacs.4c08580. Epub 2024 Aug 20. J Am Chem Soc. 2024. PMID: 39162019 Free PMC article.
-
The Dynamic Range of Acidity: Tracking Rules for the Unidirectional Penetration of Cellular Compartments.Chembiochem. 2022 Aug 3;23(15):e202200192. doi: 10.1002/cbic.202200192. Epub 2022 May 24. Chembiochem. 2022. PMID: 35535626 Free PMC article.
References
-
- Agrawal A. and Steigmann DJ (2009) Modeling protein-mediated morphology in biomembranes. Biomech. Model. Mechanobioi 8, 371–379. - PubMed
-
- Aguet F, Upadhyayula S, Gaudin R, Chou Y, Cocucci E, He K, Chen B-C, Mosaliganti K, Pasham M, Skillern W, Legant WR, Liu T-L, Findlay G, Marino E, Danuser G, Megason S, Betzig E. and Kirchhausen T. (2016) Membrane dynamics of dividing cells imaged by lattice light-sheet microscopy. Mol. Biol. Cell 27, 3418–3435. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

