Gene expression profiles of diabetic kidney disease and neuropathy in eNOS knockout mice: Predictors of pathology and RAS blockade effects
- PMID: 33788970
- PMCID: PMC10202027
- DOI: 10.1096/fj.202002387R
Gene expression profiles of diabetic kidney disease and neuropathy in eNOS knockout mice: Predictors of pathology and RAS blockade effects
Abstract
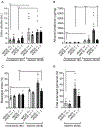
Diabetic kidney disease (DKD) and diabetic peripheral neuropathy (DPN) are two common diabetic complications. However, their pathogenesis remains elusive and current therapies are only modestly effective. We evaluated genome-wide expression to identify pathways involved in DKD and DPN progression in db/db eNOS-/- mice receiving renin-angiotensin-aldosterone system (RAS)-blocking drugs to mimic the current standard of care for DKD patients. Diabetes and eNOS deletion worsened DKD, which improved with RAS treatment. Diabetes also induced DPN, which was not affected by eNOS deletion or RAS blockade. Given the multiple factors affecting DKD and the graded differences in disease severity across mouse groups, an automatic data analysis method, SOM, or self-organizing map was used to elucidate glomerular transcriptional changes associated with DKD, whereas pairwise bioinformatic analysis was used for DPN. These analyses revealed that enhanced gene expression in several pro-inflammatory networks and reduced expression of development genes correlated with worsening DKD. Although RAS treatment ameliorated the nephropathy phenotype, it did not alter the more abnormal gene expression changes in kidney. Moreover, RAS exacerbated expression of genes related to inflammation and oxidant generation in peripheral nerves. The graded increase in inflammatory gene expression and decrease in development gene expression with DKD progression underline the potentially important role of these pathways in DKD pathogenesis. Since RAS blockers worsened this gene expression pattern in both DKD and DPN, it may partly explain the inadequate therapeutic efficacy of such blockers.
Keywords: RAS blockade; diabetic kidney disease; diabetic peripheral neuropathy; genome-wide expression; self-organizing map.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of Interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures




References
-
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, and Shahinian V (2019) US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 73, A7–A8 - PMC - PubMed
-
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, and Viswanathan V (2019) Diabetic neuropathy. Nat Rev Dis Primers 5, 41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

