Mitochondria antioxidant protection against cardiovascular dysfunction programmed by early-onset gestational hypoxia
- PMID: 33788974
- PMCID: PMC7612077
- DOI: 10.1096/fj.202002705R
Mitochondria antioxidant protection against cardiovascular dysfunction programmed by early-onset gestational hypoxia
Abstract
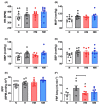
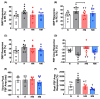
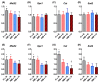
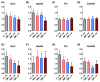
Mitochondria-derived oxidative stress during fetal development increases cardiovascular risk in adult offspring of pregnancies complicated by chronic fetal hypoxia. We investigated the efficacy of the mitochondria-targeted antioxidant MitoQ in preventing cardiovascular dysfunction in adult rat offspring exposed to gestational hypoxia, integrating functional experiments in vivo, with those at the isolated organ and molecular levels. Rats were randomized to normoxic or hypoxic (13%-14% O2 ) pregnancy ± MitoQ (500 μM day-1 ) in the maternal drinking water. At 4 months of age, one cohort of male offspring was chronically instrumented with vascular catheters and flow probes to test in vivo cardiovascular function. In a second cohort, the heart was isolated and mounted onto a Langendorff preparation. To establish mechanisms linking gestational hypoxia with cardiovascular dysfunction and protection by MitoQ, we quantified the expression of antioxidant system, β-adrenergic signaling, and calcium handling genes in the fetus and adult, in frozen tissues from a third cohort. Maternal MitoQ in hypoxic pregnancy protected offspring against increased α1 -adrenergic reactivity of the cardiovascular system, enhanced reactive hyperemia in peripheral vascular beds, and sympathetic dominance, hypercontractility and diastolic dysfunction in the heart. Inhibition of Nfe2l2-mediated oxidative stress in the fetal heart and preservation of calcium regulatory responses in the hearts of fetal and adult offspring link molecular mechanisms to the protective actions of MitoQ treatment of hypoxic pregnancy. Therefore, these data show the efficacy of MitoQ in buffering mitochondrial stress through NADPH-induced oxidative damage and the prevention of programmed cardiovascular disease in adult offspring of hypoxic pregnancy.
Keywords: IUGR; cardiovascular; fetus; hypoxia; mitochondria; oxidative stress; programming.
© 2021 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
MPM and RAJ Smith are authors on patents related to this work. The authors have stated explicitly that there are no other conflicts of interest in connection with this article.
Figures

References
-
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. - PubMed
-
- Dunbar SB, Khavjou OA, Bakas T, et al. Projected costs of informal caregiving for cardiovascular disease: 2015 to 2035 a policy statement from the American Heart Association. Circulation. 2018;137:e558–e577. - PubMed
-
- Giussani DA, Davidge ST. Developmental programming of car-diovascular disease by prenatal hypoxia. J Dev Orig Health Dis. 2013;4:328–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

