Cochlear Implantation and Other Treatments in Single-Sided Deafness and Asymmetric Hearing Loss: Results of a National Multicenter Study Including a Randomized Controlled Trial
- PMID: 33789270
- PMCID: PMC8686720
- DOI: 10.1159/000514085
Cochlear Implantation and Other Treatments in Single-Sided Deafness and Asymmetric Hearing Loss: Results of a National Multicenter Study Including a Randomized Controlled Trial
Abstract
Introduction: Cochlear implantation is a recent approach proposed to treat single-sided deafness (SSD) and asymmetric hearing loss (AHL). Several cohort studies showed its effectiveness on tinnitus and variable results on binaural hearing. The main objective of this study is to assess the outcomes of cochlear implantation and other treatment options in SSD/AHL on quality of life.
Methods: This prospective multicenter study was conducted in 7 tertiary university hospitals and included an observational cohort study of SSD/AHL adult patients treated using contralateral routing of the signal (CROS) hearing aids or bone-anchored hearing systems (BAHSs) or who declined all treatments, and a randomized controlled trial in subjects treated by cochlear implantation, after failure of CROS and BAHS trials. In total, 155 subjects with SSD or AHL, with or without associated tinnitus, were enrolled. After 2 consecutive trials with CROS hearing aids and BAHSs on headband, all subjects chose any of the 4 treatment options (abstention, CROS, BAHS, or cochlear implant [CI]). The subjects who opted for a CI were randomized between 2 arms (CI vs. initial observation). Six months after the treatment choice, quality of life was assessed using both generic (EuroQoL-5D, EQ-5D) and auditory-specific quality-of-life indices (Nijmegen Cochlear implant Questionnaire [NCIQ] and Visual Analogue Scale [VAS] for tinnitus severity). Performances for speech-in-noise recognition and localization were measured as secondary outcomes.
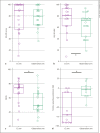
Results: CROS was chosen by 75 subjects, while 51 opted for cochlear implantation, 18 for BAHSs, and 11 for abstention. Six months after treatment, both EQ-5D VAS and auditory-specific quality-of-life indices were significantly better in the "CI" arm versus "observation" arm. The mean effect of the CI was particularly significant in subjects with associated severe tinnitus (mean improvement of 20.7 points ± 19.7 on EQ-5D VAS, 20.4 ± 12.4 on NCIQ, and 51.4 ± 35.4 on tinnitus). No significant effect of the CI was found on binaural hearing results. Before/after comparisons showed that the CROS and BAHS also improved significantly NCIQ scores (for CROS: +7.7, 95% confidence interval [95% CI] = [4.5; 10.8]; for the BAHS: +14.3, 95% CI = [7.9; 20.7]).
Conclusion: Cochlear implantation leads to significant improvements in quality of life in SSD and AHL patients, particularly in subjects with associated severe tinnitus, who are thereby the best candidates to an extension of CI indications.
Keywords: Asymmetric hearing loss; Bone-anchored hearing system; Cochlear implants; Contralateral routing of the signal hearing Aids; Quality of life.
The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32((1)):39–47. - PubMed
-
- Arts RAGJ, George ELJ, Griessner A, Zierhofer C, Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single-sided deafness: a prospective clinical trial: part I. Audiol Neurootol. 2015;20:294–313. - PubMed
-
- Baguley DM, Atlas MD. Cochlear implants and tinnitus. Prog Brain Res. 2007;166:347–55. - PubMed
-
- Baguley DM, Bird J, Humphriss RL, Prevost AT. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol. 2006;31((1)):6–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

