Viral Respiratory Pathogens and Lung Injury
- PMID: 33789928
- PMCID: PMC8142519
- DOI: 10.1128/CMR.00103-20
Viral Respiratory Pathogens and Lung Injury
Abstract
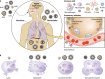
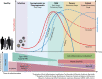
Several viruses target the human respiratory tract, causing different clinical manifestations spanning from mild upper airway involvement to life-threatening acute respiratory distress syndrome (ARDS). As dramatically evident in the ongoing SARS-CoV-2 pandemic, the clinical picture is not always easily predictable due to the combined effect of direct viral and indirect patient-specific immune-mediated damage. In this review, we discuss the main RNA (orthomyxoviruses, paramyxoviruses, and coronaviruses) and DNA (adenoviruses, herpesviruses, and bocaviruses) viruses with respiratory tropism and their mechanisms of direct and indirect cell damage. We analyze the thin line existing between a protective immune response, capable of limiting viral replication, and an unbalanced, dysregulated immune activation often leading to the most severe complication. Our comprehension of the molecular mechanisms involved is increasing and this should pave the way for the development and clinical use of new tailored immune-based antiviral strategies.
Keywords: COVID-19; SARS-CoV-2; respiratory tract; viral infections.
Copyright © 2021 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

