The Rhesus Macaque Serves As a Model for Human Lateral Branch Nephrogenesis
- PMID: 33789950
- PMCID: PMC8259676
- DOI: 10.1681/ASN.2020101459
The Rhesus Macaque Serves As a Model for Human Lateral Branch Nephrogenesis
Abstract
Background: Most nephrons are added in late gestation. Truncated extrauterine nephrogenesis in premature infants results in fewer nephrons and significantly increased risk for CKD in adulthood. To overcome the ethical and technical difficulties associated with studies of late-gestation human fetal kidney development, third-trimester rhesus macaques served as a model to understand lateral branch nephrogenesis (LBN) at the molecular level.
Methods: Immunostaining and 3D rendering assessed morphology. Single-cell (sc) and single-nucleus (sn) RNA-Seq were performed on four cortically enriched fetal rhesus kidneys of 129-131 days gestational age (GA). An integrative bioinformatics strategy was applied across single-cell modalities, species, and time. RNAScope validation studies were performed on human archival tissue.
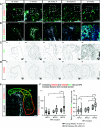
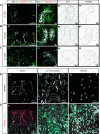
Results: Third-trimester rhesus kidney undergoes human-like LBN. scRNA-Seq of 23,608 cells revealed 37 transcriptionally distinct cell populations, including naïve nephron progenitor cells (NPCs), with the prior noted marker genes CITED1, MEOX1, and EYA1 (c25). These same populations and markers were reflected in snRNA-Seq of 5972 nuclei. Late-gestation rhesus NPC markers resembled late-gestation murine NPC, whereas early second-trimester human NPC markers aligned to midgestation murine NPCs. New, age-specific rhesus NPCs (SHISA8) and ureteric buds (POU3F4 and TWIST) predicted markers were verified in late-gestation human archival samples.
Conclusions: Rhesus macaque is the first model of bona fide LBN, enabling molecular studies of late gestation, human-like nephrogenesis. These molecular findings support the hypothesis that aging nephron progenitors have a distinct molecular signature and align to their earlier human counterparts, with unique markers highlighting LBN-specific progenitor maturation.
Keywords: chronic kidney disease; lateral branch nephrogenesis; nephrogenesis; prematurity.
Copyright © 2021 by the American Society of Nephrology.
Figures




Comment in
-
Monkeying about with Nephron Formation.J Am Soc Nephrol. 2021 May 3;32(5):1011-1013. doi: 10.1681/ASN.2021030320. Epub 2021 Apr 7. J Am Soc Nephrol. 2021. PMID: 33827903 Free PMC article. No abstract available.
References
-
- Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D: Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991. - PubMed
-
- Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992. - PubMed
-
- Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K: Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 16: 2557–2564, 2005. - PubMed
-
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE: Human nephron number: Implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011. - PubMed
-
- Barker DJ: The intrauterine environment and adult cardiovascular disease. Ciba Found Symp 156: 3–10, discussion 10–16, 1991. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

