Targeting acute myeloid leukemia dependency on VCP-mediated DNA repair through a selective second-generation small-molecule inhibitor
- PMID: 33790022
- PMCID: PMC8672851
- DOI: 10.1126/scitranslmed.abg1168
Targeting acute myeloid leukemia dependency on VCP-mediated DNA repair through a selective second-generation small-molecule inhibitor
Abstract
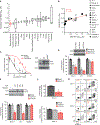
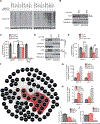
The development and survival of cancer cells require adaptive mechanisms to stress. Such adaptations can confer intrinsic vulnerabilities, enabling the selective targeting of cancer cells. Through a pooled in vivo short hairpin RNA (shRNA) screen, we identified the adenosine triphosphatase associated with diverse cellular activities (AAA-ATPase) valosin-containing protein (VCP) as a top stress-related vulnerability in acute myeloid leukemia (AML). We established that AML was the most responsive disease to chemical inhibition of VCP across a panel of 16 cancer types. The sensitivity to VCP inhibition of human AML cell lines, primary patient samples, and syngeneic and xenograft mouse models of AML was validated using VCP-directed shRNAs, overexpression of a dominant-negative VCP mutant, and chemical inhibition. By combining mass spectrometry-based analysis of the VCP interactome and phospho-signaling studies, we determined that VCP is important for ataxia telangiectasia mutated (ATM) kinase activation and subsequent DNA repair through homologous recombination in AML. A second-generation VCP inhibitor, CB-5339, was then developed and characterized. Efficacy and safety of CB-5339 were validated in multiple AML models, including syngeneic and patient-derived xenograft murine models. We further demonstrated that combining DNA-damaging agents, such as anthracyclines, with CB-5339 treatment synergizes to impair leukemic growth in an MLL-AF9-driven AML murine model. These studies support the clinical testing of CB-5339 as a single agent or in combination with standard-of-care DNA-damaging chemotherapy for the treatment of AML.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures





References
-
- Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Dohner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA, Dohner H, Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med 377, 454–464 (2017). - PMC - PubMed
-
- DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, Swords R, Collins RH, Mannis GN, Pollyea DA, Donnellan W, Fathi AT, Pigneux A, Erba HP, Prince GT, Stein AS, Uy GL, Foran JM, Traer E, Stuart RK, Arellano ML, Slack JL, Sekeres MA, Willekens C, Choe S, Wang H, Zhang V, Yen KE, Kapsalis SM, Yang H, Dai D, Fan B, Goldwasser M, Liu H, Agresta S, Wu B, Attar EC, Tallman MS, Stone RM, Kantarjian HM, Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med 378, 2386–2398 (2018). - PubMed
-
- Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, DeAngelo DJ, Levine RL, Flinn IW, Kantarjian HM, Collins R, Patel MR, Frankel AE, Stein A, Sekeres MA, Swords RT, Medeiros BC, Willekens C, Vyas P, Tosolini A, Xu Q, Knight RD, Yen KE, Agresta S, de Botton S, Tallman MS, Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130, 722–731 (2017). - PMC - PubMed
-
- Ellis LM, Hicklin DJ, Resistance to Targeted Therapies: Refining Anticancer Therapy in the Era of Molecular Oncology. Clin Cancer Res 15, 7471–7478 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

