Efficacy of ketamine in relieving neuropathic pain: a systematic review and meta-analysis of animal studies
- PMID: 33790195
- PMCID: PMC8374709
- DOI: 10.1097/j.pain.0000000000002231
Efficacy of ketamine in relieving neuropathic pain: a systematic review and meta-analysis of animal studies
Abstract
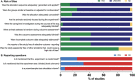
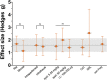
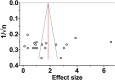
In humans, proof of long-term efficacy of ketamine treatment in neuropathic pain is lacking. To improve our understanding of ketamine behavior under various administration conditions, we performed a systematic review and meta-analyses of controlled studies on the efficacy of ketamine in mice and rats with a disease model of nerve injury on relief of allodynia. Searches in PubMed and EMBASE identified 31 unique studies. Four meta-analyses were conducted. The first analysis included 19 comparisons on a single ketamine dose and measurement of effect within 3 hours of dosing and showed an appreciable effect (standardized mean difference 1.6, 95% confidence interval 1.1-2.1). Subgroup analyses showed no effect of species, administration route, or dose. A single administration was insufficient to sustain relief of allodynia at 24 or 72 hours after dosing, as observed in our second analysis (7 comparisons) with similar effects in ketamine-treated and control animals. Chronic ketamine administration (9 comparisons) caused profound relief of allodynia when tested during ketamine exposure (effect size 5.1, 3.7-6.5). The final analysis (6 comparisons) showed that chronic administration caused a slow loss of relief of allodynia with 70% loss of effect 24 days after end of treatment. No subgroups analyses were possible in the last 3 meta-analyses due to small group sizes. These results indicate long-term ketamine anti-allodynic effects after chronic exposure (>3 days) but not after a single administration. Given several limitations, extrapolation of the animal data to the human condition is tenuous.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



References
-
- Chaplan SR, Malmberg AB, Yaksh TL. Effect of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther 1997;280:829–38. - PubMed
-
- Christoph T, Schiene K, Engelberger W, Parsons CG, Chizh BA. The antiallodynic effect of NMDA antagonists in neuropathic pain outlasts the duration of the in vivo NMDA antagonism. Neuropharmacol 2006;51:12–7. - PubMed
-
- Claudino R, Nones C, Araya E, Chicorro J. Analgesic effects of intranasal ketamine in rat models of facial pain. J Oral Facial Pain Headache 2018;32:238–46. - PubMed
-
- Dahan A, Olofsen E, Sigtermans M, Noppers I, Niesters M, Aarts L, Bauer M, Sarton E. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief of chronic pain. Eur J Pain 2011;15:258–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

