An amygdala circuit that suppresses social engagement
- PMID: 33790466
- PMCID: PMC9251649
- DOI: 10.1038/s41586-021-03413-6
An amygdala circuit that suppresses social engagement
Abstract
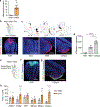
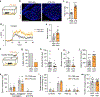
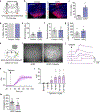
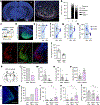
Innate social behaviours, such as mating and fighting, are fundamental to animal reproduction and survival1. However, social engagements can also put an individual at risk2. Little is known about the neural mechanisms that enable appropriate risk assessment and the suppression of hazardous social interactions. Here we identify the posteromedial nucleus of the cortical amygdala (COApm) as a locus required for the suppression of male mating when a female mouse is unhealthy. Using anatomical tracing, functional imaging and circuit-level epistatic analyses, we show that suppression of mating with an unhealthy female is mediated by the COApm projections onto the glutamatergic population of the medial amygdalar nucleus (MEA). We further show that the role of the COApm-to-MEA connection in regulating male mating behaviour relies on the neuromodulator thyrotropin-releasing hormone (TRH). TRH is expressed in the COApm, whereas the TRH receptor (TRHR) is found in the postsynaptic MEA glutamatergic neurons. Manipulating neural activity of TRH-expressing neurons in the COApm modulated male mating behaviour. In the MEA, activation of the TRHR pathway by ligand infusion inhibited mating even towards healthy female mice, whereas genetic ablation of TRHR facilitated mating with unhealthy individuals. In summary, we reveal a neural pathway that relies on the neuromodulator TRH to modulate social interactions according to the health status of the reciprocating individual. Individuals must balance the cost of social interactions relative to the benefit, as deficits in the ability to select healthy mates may lead to the spread of disease.
Conflict of interest statement
The authors declare no competing financial interests.
Figures










References
-
- Tinbergen N The study of instinct. New York, NY: Claredon Press/Oxford University Press; (1951).
-
- Altizer S et al. Social Organization and Parasite Risk in Mammals: Integrating Theory and Empirical Studies. Annual Review of Ecology, Evolution, and Systematics 34, 517–547 (2003).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

