Treatment of Metastatic Extramammary Paget Disease with Combination Ipilimumab and Nivolumab: A Case Report
- PMID: 33790764
- PMCID: PMC7983595
- DOI: 10.1159/000514345
Treatment of Metastatic Extramammary Paget Disease with Combination Ipilimumab and Nivolumab: A Case Report
Abstract
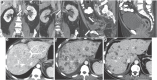
Metastatic primary cutaneous extramammary Paget disease (EMPD) is a rare clinical entity with a 5-year survival <10% and no standard therapy. We report the first case to our knowledge of metastatic EMPD with treatment response to checkpoint inhibitor immunotherapy. The patient had diffusely metastatic disease and previously progressed on cytotoxic chemotherapy and a molecularly targeted agent. Treatment with four cycles of ipilimumab 1 mg/kg plus nivolumab 3 mg/kg resulted in a durable partial response lasting 7 months. Analysis of metastatic tumor tissue failed to identify known predictors of treatment response to immune checkpoint inhibitors, such as high PD-L1 expression, high tumor mutation burden, or microsatellite instability. These findings support further investigation of immune checkpoint inhibition for the management of metastatic EMPD, which currently has an abysmal prognosis and no standard therapies.
Keywords: Extramammary Paget disease; Immune checkpoint blockade; Immunotherapy; Ipilimumab; Nivolumab.
Copyright © 2021 by S. Karger AG, Basel.
Conflict of interest statement
All authors affiliated with Memorial Sloan Kettering Cancer Center report institutional support during the conduct of the study from NIH/NCI Cancer Center Support Grant P30 CA008748, the Pulver family in the form of a charitable donation to Dr. Travis Hollmann, and center support from the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center. Dr. Ghafoor and Dr. Chen report no additional disclosures. Dr. Guercio reports support from NIH/NCI Grant T32-CA009207, honoraria from Medscape, and institutional research funding from Bristol-Myers Squibb, Genentech, Eli Lilly, Pfizer, and Sanofi, outside the submitted work. Dr. Hollmann reports research funding from General Electric, outside the submitted work. Dr. Iyer reports grants and personal fees from Mirati Therapeutics, grants from Novartis, grants from DeBioPharm, grants and personal fees from Janssen, outside the submitted work. Dr. Lacouture reports royalties from Legacy Healthcare Services, Apricity Health, LLC, Azitra, Inc., Deciphera, Galderma Research and Development, Johnson and Johnson, NCODA, Novocure Inc., Kyowa Kirin, Inc., Loxo, Merck Sharp and Dohme Corporation, Janssen Research & Development, LLC, Menlo Therapeutics, Novartis Pharmaceuticals Corporation, QED Therapeutics, F. Hoffmann-La Roche AG, Amgen Inc., AstraZeneca Pharmceuticals LP, Genentech, Inc., Seattle Genetics, Lutris, Paxman Coolers, OnQuality Pharmaceuticals Ltd., Takeda Millenium, outside the submitted work. Dr. Rossi reports personal fees from Regeneron and Biofrontera for advisory board positions, outside the submitted work. Dr. Busam reports royalties from Elsevier. Dr. Janjigian reports stock options from Rgenix, consulting/advisory for AstraZeneca, consulting/advisory for Daiichi Sankyo, research funding from ONO Pharma, consulting/advisory and research funding from Merck & Co Inc., consulting/advisory and research funding from Bristol-Myers Squibb, research funding from Boehringer Ingelheim, research funding from Bayer, research funding from Genentech/Roche, consulting/advisory for Merck Serono, consulting/advisory for Pfizer, consulting/advisory for Imugene, consulting/advisory and research funding from Eli Lilly, speaking fees from ASCO, consulting/advisory for Michael J. Hennessy Associates, consulting/advisory for Paradigm Medical Communications LLC, consulting/advisory for Zymeworks Inc., consulting/advisory for Jounce Therapeutics, and consulting/advisory for Seattle Genetics, outside the submitted work. Dr. Jhaveri reports consulting/advisory role and expenses from AstraZeneca, consulting/advisory, expenses, and institutional research funding from Pfizer, consulting/advisory role and institutional research funding from Novartis, consulting/advisory role and expenses from Taiho Pharmaceutical, advisory board member role for Juno Therapeutics, consulting/advisory role and institutional research funding from ADC Therapeutics, advisory board member role for and institutional research funding from Genentech, consulting/advisory role for Synthon, consulting/advisory role, speaker fees, and expenses from Intellisphere, consultant/advisory role for Spectrum Pharmaceuticals, institutional research funding from Zymeworks, consulting/advisory role and institutional research funding from Eli Lilly and Co, institutional research funding from Novita Pharmaceuticals, institutional research funding from Debio Pharmaceuticals, consulting/advisory role for Abbvie, consulting/advisory role for Bristol-Myers Squibb, consulting/advisory role for and expenses from Jounce Therapeutics, consulting/advisory role for Spectrum Pharmaceuticals, institutional research funding from Clovis Oncology, institutional research funding from Immunomedics, and institutional research funding from Puma Biotechnology, outside the submitted work. Dr. Feldman reports research funding and drug supply from Seattle Genetics, research funding from Novartis, research funding and drug supply for clinical trial from Astellas, research funding from Decibel, outside the submitted work. Dr. Bajorin reports honoraria from Merck Sharp & Dohme, consulting/advisory role, expenses, and institutional research funding from Genentech/Roche, consulting/advisory role, expenses, and institutional research funding from Bristol-Myers Squibb, consulting/advisory role for Fidia Farmaceutici, consulting/advisory role for Pfizer, institutional research funding from AstraZeneca, consulting/advisory role and expenses from Eli Lilly, grants, consulting/advisory role, expenses, and institutional research funding from Merck, grants, consulting/advisory role, and institutional research funding from Novartis, consulting/advisory role and expenses from UroGen Pharma, consulting/advisory role for Roche, consulting/advisory role for EMD Serono, institutional research funding from Seattle Genetics, and institutional research funding from Astellas Pharma, outside the submitted work. Dr. Rosenberg reports honoraria, consulting/advisory role, and expenses from Bristol-Myers Squibb, institutional research funding, honoraria, and consulting/advisory role from AstraZeneca, honoraria from Chugai Pharma, consulting/advisory role for Lilly, consulting/advisory role for Merck, consulting/advisory role and institutional research funding from Agensys, consulting/advisory role, expenses, and institutional research funding from Genentech/Roche, consulting/advisory role for Sanofi, consulting/advisory role for EMD Serono, consulting/advisory role and institutional research funding from Seattle Genetics, consulting/advisory role and institutional research funding from Bayer, consulting/advisory role for Inovio Pharmaceuticals, consulting/advisory role for BioClin Therapeutics, consulting/advisory role and institutional research funding from QED Therapeutics, consulting/advisory role for Adicet Bio, consulting/advisory role for Sensei Biotherapeutics, consulting/advisory role for Fortress Biotech, consulting/advisory role for Pharmacyclics, consulting/advisory role for Western Oncolytics, consulting/advisory role for GlaxoSmithKline, consulting/advisory role for Janssen Oncology, consulting/advisory role and institutional research funding from Astellas Pharma, consulting/advisory role for Boehringer Ingelheim, consulting/advisory role and institutional research funding from Mirati Therapeutics, institutional research funding from Oncogenex, institutional research funding from Novartis, institutional research funding from Viralytics, institutional research funding from Incyte, institutional research funding from Jounce Therapeutics, stock/ownership interests from Illumina, consulting/advisory role for AstraZeneca/MedImmune, and consulting for Pfizer, outside the submitted work. In addition, Dr. Rosenberg has a patent Predictor of platinum sensitivity owned by Memorial Sloan Kettering Cancer Center. Dr. Hollmann reports research funding from General Electric, outside the submitted work. Dr. Funt reports stock or other ownership interests from Allogene Therapeutics, Urogen, Neogene Therapeutics, Vaxigene, Kronos Bio, and Vida Ventures, research funding and travel expenses from AstraZeneca, research funding from Genentech/Roche, consulting/advisory role for Decibel, and consulting/advisory role for Immunai, outside the submitted work. Dr. Kidwai and Dr. Assis have nothing to disclose.
Figures

References
-
- van der Linden M, Meeuwis KA, Bulten J, Bosse T, van Poelgeest MI, de Hullu JA. Paget disease of the vulva. Crit Rev Oncol Hematol. 2016;101:60–74. - PubMed
-
- Shah MA, Janjigian YY, Stoller R, Shibata S, Kemeny M, Krishnamurthi S, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J Clin Oncol. 2015;33((33)):3874–9. - PubMed
-
- US Food and Drug Administration FDA unveils a streamlined path for the authorization of tumor profiling tests alongside its latest product action [updated 2017 Nov 15; cited 2020 Nov 14] Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm585347.htm.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

