Stroke Treatment With PAR-1 Agents to Decrease Hemorrhagic Transformation
- PMID: 33790846
- PMCID: PMC8005555
- DOI: 10.3389/fneur.2021.593582
Stroke Treatment With PAR-1 Agents to Decrease Hemorrhagic Transformation
Abstract
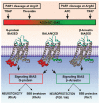
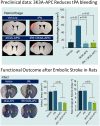
Ischemic stroke is the most widespread cause of disability and a leading cause of death in developed countries. To date, the most potent approved treatment for acute stroke is recanalization therapy with thrombolytic drugs such as tissue plasminogen activator (rt-PA or tPA) or endovascular mechanical thrombectomy. Although tPA and thrombectomy are widely available in the United States, it is currently estimated that only 10-20% of stroke patients get tPA treatment, in part due to restrictive selection criteria. Recently, however, tPA and thrombectomy selection criteria have loosened, potentially allowing more patients to qualify. The relatively low rate of treatment may also reflect the perceived risk of brain hemorrhage following treatment with tPA. In translational research and a single patient study, protease activated receptor 1 (PAR-1) targeted therapies given along with thrombolysis and thrombectomy appear to reduce hemorrhagic transformation after recanalization. Such adjuncts may likely enhance the availability of recanalization and encourage more physicians to use the recently expanded selection criteria for applying recanalization therapies. This narrative review discusses stroke therapies, the role of hemorrhagic transformation in producing poor outcomes, and presents the data suggesting that PAR-1 acting agents show promise for decreasing hemorrhagic transformation and improving outcomes.
Keywords: activated protein C; bleeding; hemorrhagic transformation; intracranial hemorrhage; ischemic stroke; stroke therapy; thrombectomy; tissue plasminogen activator.
Copyright © 2021 Lyden, Pryor, Minigh, Davis, Griffin, Levy and Zlokovic.
Conflict of interest statement
KP is employed by ZZ Biotech. JG, HL, JM, and BZ are paid consultants to ZZ Biotech. PL has received research funding from NIH to conduct trials of 3K3A-APC; received royalties from sales of the book Thrombolytic Therapy for Acute Ischemic Stroke, 3rd Edition; and received fees for occasional expert witness testimony. The Scripps Research Institute has intellectual property related to the topic of this review. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics-2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. (2006) 113:e85–151. 10.1161/CIRCULATIONAHA.105.171600 - DOI - PubMed
-
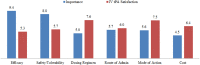
- Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, et al. Final results of the RHAPSODY trial: a multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol. (2019) 85:125–36. 10.1002/ana.25383 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

