Malawian children with chest-indrawing pneumonia with and without comorbidities or danger signs
- PMID: 33791095
- PMCID: PMC7979154
- DOI: 10.7189/jogh.11.04016
Malawian children with chest-indrawing pneumonia with and without comorbidities or danger signs
Abstract
Background: Children with comorbidities or danger signs are often excluded from trials evaluating pneumonia treatment.
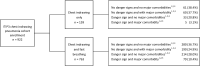
Methods: We sought to investigate whether the percentage of children with chest-indrawing pneumonia cured at Day 14 was lower among those with HIV infection or exposure, malaria, moderate or severe acute malnutrition, or anemia enrolled in a prospective observational cohort study than among children without these comorbidities enrolled in a concurrent prospective randomized controlled trial evaluating duration of amoxicillin treatment in Lilongwe, Malawi.
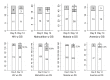
Results: Children with chest-indrawing pneumonia and comorbidities but without danger signs did not have statistically significant higher treatment failure rates by Day 6 than those in the chest-indrawing pneumonia clinical trial. However, children with chest-indrawing pneumonia and HIV infection or exposure, malaria, or moderate or severe acute malnutrition had higher rates of not being clinically cured at Day 14 when compared to children without these comorbidities (adjusted differences ranging from 7.7% to 17.0%). Furthermore, among children without danger signs at enrollment, but with HIV infection or HIV exposure or moderate or severe acute malnutrition, 12.5% and 15.6% respectively were not clinically cured at Day 14 even though they were without treatment failure by Day 6.
Conclusions: More intensive follow-up of children with chest-indrawing pneumonia and comorbidities who do not have danger signs may be beneficial.
Copyright © 2021 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Competing interests: The authors completed the ICMJE Unified Competing Interest form (available upon request from the corresponding author), and declare no conflicts of interest.
Figures
References
-
- UNICEF Pneumonia. Available: https://data.unicef.org/topic/child-health/pneumonia/. Accessed: 2 October 2020.
-
- Ginsburg AS, May SJ, Nkwopara E, Ambler G, McCollum ED, Mvalo T, et al. Methods for conducting a double-blind randomized controlled clinical trial of three days versus five days of amoxicillin dispersible tablets for chest indrawing childhood pneumonia among children two to 59 months of age in Lilongwe, Malawi: a study protocol. BMC Infect Dis. 2018;18:476. 10.1186/s12879-018-3379-z - DOI - PMC - PubMed
-
- Ginsburg AS, May S, Nkwopara E, Ambler G, McCollum ED, Mvalo T, et al. Clinical Outcomes of Pneumonia and Other Comorbidities in Children Aged 2-59 Months in Lilongwe, Malawi: Protocol for the Prospective Observational Study “Innovative Treatments in Pneumonia”. JMIR Res Protoc. 2019;8:e13377. 10.2196/13377 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
