Recent Advances in the Synthesis of High Boron-Loaded Nucleic Acids for BNCT
- PMID: 33791278
- PMCID: PMC8005562
- DOI: 10.3389/fchem.2021.619052
Recent Advances in the Synthesis of High Boron-Loaded Nucleic Acids for BNCT
Abstract
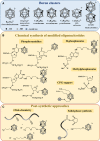
Boron clusters attract considerable attention as promising therapeutic tools for boron neutron capture therapy (BNCT). They combine high boron content with high chemical and biological stability, biorthogonality, and low toxicity. The development of oligonucleotide-based constructs and nucleic acid-like molecules, such as oligomeric phosphate diesters, bearing one or multiple boron clusters permits to create potential high boron-loaded agents for BNCT with good bioavailability, specifically interacting with nucleic acids inside the cell. Here, we shortly review the strategies and solutions in the design of oligonucleotide conjugates with boron clusters in light of the requirements for effective BNCT and future prospects of their practical use.
Keywords: BNCT; boron cluster; boron neutron capture therapy; conjugation method; oligonucleotide conjugate; pre- and post-synthetic modification.
Copyright © 2021 Novopashina, Vorobyeva and Venyaminova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

