Microbial Degradation of Naphthalene and Substituted Naphthalenes: Metabolic Diversity and Genomic Insight for Bioremediation
- PMID: 33791281
- PMCID: PMC8006333
- DOI: 10.3389/fbioe.2021.602445
Microbial Degradation of Naphthalene and Substituted Naphthalenes: Metabolic Diversity and Genomic Insight for Bioremediation
Abstract
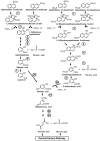
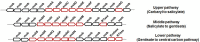
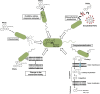
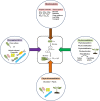
Low molecular weight polycyclic aromatic hydrocarbons (PAHs) like naphthalene and substituted naphthalenes (methylnaphthalene, naphthoic acids, 1-naphthyl N-methylcarbamate, etc.) are used in various industries and exhibit genotoxic, mutagenic, and/or carcinogenic effects on living organisms. These synthetic organic compounds (SOCs) or xenobiotics are considered as priority pollutants that pose a critical environmental and public health concern worldwide. The extent of anthropogenic activities like emissions from coal gasification, petroleum refining, motor vehicle exhaust, and agricultural applications determine the concentration, fate, and transport of these ubiquitous and recalcitrant compounds. Besides physicochemical methods for cleanup/removal, a green and eco-friendly technology like bioremediation, using microbes with the ability to degrade SOCs completely or convert to non-toxic by-products, has been a safe, cost-effective, and promising alternative. Various bacterial species from soil flora belonging to Proteobacteria (Pseudomonas, Pseudoxanthomonas, Comamonas, Burkholderia, and Novosphingobium), Firmicutes (Bacillus and Paenibacillus), and Actinobacteria (Rhodococcus and Arthrobacter) displayed the ability to degrade various SOCs. Metabolic studies, genomic and metagenomics analyses have aided our understanding of the catabolic complexity and diversity present in these simple life forms which can be further applied for efficient biodegradation. The prolonged persistence of PAHs has led to the evolution of new degradative phenotypes through horizontal gene transfer using genetic elements like plasmids, transposons, phages, genomic islands, and integrative conjugative elements. Systems biology and genetic engineering of either specific isolates or mock community (consortia) might achieve complete, rapid, and efficient bioremediation of these PAHs through synergistic actions. In this review, we highlight various metabolic routes and diversity, genetic makeup and diversity, and cellular responses/adaptations by naphthalene and substituted naphthalene-degrading bacteria. This will provide insights into the ecological aspects of field application and strain optimization for efficient bioremediation.
Keywords: biodegradation; bioremediation; cellular responses and evolution; gene transfer mechanisms; genetic diversity; metabolic pathways; naphthalene; substituted naphthalenes.
Copyright © 2021 Mohapatra and Phale.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Annweiler E., Richnow H. H., Antranikian G., Hebenbrock S., Garms C., Franke S., et al. (2000). Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophile Bacillus thermoleovorans. Appl. Environ. Microbiol. 66 518–523. 10.1128/aem.66.2.518-523.2000 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

