Coronavirus-Induced Host Cubic Membranes and Lipid-Related Antiviral Therapies: A Focus on Bioactive Plasmalogens
- PMID: 33791293
- PMCID: PMC8006408
- DOI: 10.3389/fcell.2021.630242
Coronavirus-Induced Host Cubic Membranes and Lipid-Related Antiviral Therapies: A Focus on Bioactive Plasmalogens
Abstract
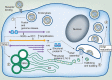
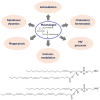
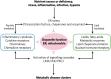
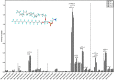
Coronaviruses have lipid envelopes required for their activity. The fact that coronavirus infection provokes the formation of cubic membranes (CM) (denoted also as convoluted membranes) in host cells has not been rationalized in the development of antiviral therapies yet. In this context, the role of bioactive plasmalogens (vinyl ether glycerophospholipids) is not completely understood. These lipid species display a propensity for non-lamellar phase formation, facilitating membrane fusion, and modulate the activity of membrane-bound proteins such as enzymes and receptors. At the organism level, plasmalogen deficiency is associated with cardiometabolic disorders including obesity and type 2 diabetes in humans. A straight link is perceived with the susceptibility of such patients to SARS-CoV-2 (severe acute respiratory syndrome-coronavirus-2) infection, the severity of illness, and the related difficulty in treatment. Based on correlations between the coronavirus-induced modifications of lipid metabolism in host cells, plasmalogen deficiency in the lung surfactant of COVID-19 patients, and the alterations of lipid membrane structural organization and composition including the induction of CM, we emphasize the key role of plasmalogens in the coronavirus (SARS-CoV-2, SARS-CoV, or MERS-CoV) entry and replication in host cells. Considering that plasmalogen-enriched lung surfactant formulations may improve the respiratory process in severe infected individuals, plasmalogens can be suggested as an anti-viral prophylactic, a lipid biomarker in SARS-CoV and SARS-CoV-2 infections, and a potential anti-viral therapeutic component of lung surfactant development for COVID-19 patients.
Keywords: COVID-19; TEM; coronavirus; cubic membrane; plasmalogen; virus-host interaction.
Copyright © 2021 Deng and Angelova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Ahmed S., Akter M. S., Roy K., Islam M. S. (2020). Role of surfactant for the treatment of alveolar cells against coronavirus (Covid-19). Ann. Res. Rev. Biol. 144:110020 10.9734/arrb/2020/v35i630233 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

