Donepezil Ameliorates Pulmonary Arterial Hypertension by Inhibiting M2-Macrophage Activation
- PMID: 33791350
- PMCID: PMC8005547
- DOI: 10.3389/fcvm.2021.639541
Donepezil Ameliorates Pulmonary Arterial Hypertension by Inhibiting M2-Macrophage Activation
Abstract
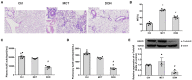
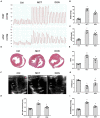
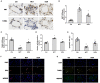
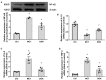
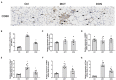
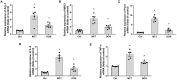
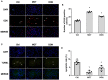
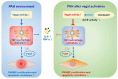
Background: The beneficial effects of parasympathetic stimulation in pulmonary arterial hypertension (PAH) have been reported. However, the specific mechanism has not been completely clarified. Donepezil, an oral cholinesterase inhibitor, enhances parasympathetic activity by inhibiting acetylcholinesterase, whose therapeutic effects in PAH and its mechanism deserve to be investigated. Methods: The PAH model was established by a single intraperitoneal injection of monocrotaline (MCT, 50 mg/kg) in adult male Sprague-Dawley rats. Donepezil was administered via intraperitoneal injection daily after 1 week of MCT administration. At the end of the study, PAH status was confirmed by echocardiography and hemodynamic measurement. Testing for acetylcholinesterase activity and cholinergic receptor expression was used to evaluate parasympathetic activity. Indicators of pulmonary arterial remodeling and right ventricular (RV) dysfunction were assayed. The proliferative and apoptotic ability of pulmonary arterial smooth muscle cells (PASMCs), inflammatory reaction, macrophage infiltration in the lung, and activation of bone marrow-derived macrophages (BMDMs) were also tested. PASMCs from the MCT-treated rats were co-cultured with the supernatant of BMDMs treated with donepezil, and then, the proliferation and apoptosis of PASMCs were evaluated. Results: Donepezil treatment effectively enhanced parasympathetic activity. Furthermore, it markedly reduced mean pulmonary arterial pressure and RV systolic pressure in the MCT-treated rats, as well as reversed pulmonary arterial remodeling and RV dysfunction. Donepezil also reduced the proliferation and promoted the apoptosis of PASMCs in the MCT-treated rats. In addition, it suppressed the inflammatory response and macrophage activation in both lung tissue and BMDMs in the model rats. More importantly, donepezil reduced the proliferation and promoted the apoptosis of PASMCs by suppressing M2-macrophage activation. Conclusion: Donepezil could prevent pulmonary vascular and RV remodeling, thereby reversing PAH progression. Moreover, enhancement of the parasympathetic activity could reduce the proliferation and promote the apoptosis of PASMCs in PAH by suppressing M2-macrophage activation.
Keywords: M2-macrophage; cholinesterase inhibitor; donepezil; pulmonary arterial hypertension; pulmonary arterial smooth muscle cells.
Copyright © 2021 Qiu, Zhang, Li, Jiang, Guo, He and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

