Bifunctional Role of the Sternohyoideus Muscle During Suction Feeding in Striped Surfperch, Embiotoca lateralis
- PMID: 33791562
- PMCID: PMC7671119
- DOI: 10.1093/iob/obaa021
Bifunctional Role of the Sternohyoideus Muscle During Suction Feeding in Striped Surfperch, Embiotoca lateralis
Abstract
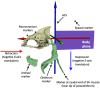
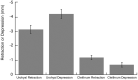
In ray-finned fishes, the sternohyoideus (SH) is among the largest muscles in the head region and, based on its size, can potentially contribute to the overall power required for suction feeding. However, the function of the SH varies interspecifically. In largemouth bass (Micropterus salmoides) and several clariid catfishes, the SH functions similarly to a stiff ligament. In these species, the SH remains isometric and transmitts power from the hypaxial musculature to the hyoid apparatus during suction feeding. Alternatively, the SH can shorten and contribute muscle power during suction feeding, a condition observed in the bluegill sunfish (Lepomis macrochirus) and one clariid catfish. An emerging hypothesis centers on SH muscle size as a predictor of function: in fishes with a large SH, the SH shortens during suction feeding, whereas in fish with a smaller SH, the muscle may remain isometric. Here, we studied striped surfperch (Embiotoca lateralis), a species in which the SH is relatively large at 8.8% of axial muscle mass compared with 4.0% for L. macrochirus and 1.7% for M. salmoides, to determine whether the SH shortens during suction feeding and is, therefore, bifunctional-both transmitting and generating power-or remains isometric and only transmits power. We measured skeletal kinematics of the neurocranium, urohyal, and cleithrum with Video Reconstruction of Moving Morphology, along with muscle strain and shortening velocity in the SH and epaxial muscles, using a new method of 3D external marker tracking. We found mean SH shortening during suction feeding strikes (n = 22 strikes from four individual E. lateralis) was 7.2 ± 0.55% (±SEM) of initial muscle length. Mean peak speed of shortening was 4.9 ± 0.65 lengths s-1, and maximum shortening speed occurred right around peak gape when peak power is generated in suction feeding. The cleithrum of E. lateralis retracts and depresses but the urohyal retracts and depresses even more, a strong indicator of a bifunctional SH capable of not only generating its own power but also transmitting hypaxial power to the hyoid. While power production in E. lateralis is still likely dominated by the axial musculature, since even the relatively large SH of E. lateralis is only 8.8% of axial muscle mass, the SH may contribute a meaningful amount of power given its continual shortening just prior to peak gape across all strikes. These results support the finding from other groups of fishes that a large SH muscle, relative to axial muscle mass, is likely to both generate and transmit power during suction feeding.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Integrative and Comparative Biology.
Figures





References
-
- Aerts P. 1991. Hyoid morphology and movements relative to abducting forces during feeding in Astatotilapia elegans (Teleostei: Cichlidae). J Morph 208:323–45. - PubMed
-
- Askew GN, Marsh RL. 1998. Optimal shortening velocity (V/Vmax) of skeletal muscle during cyclical contractions: length-force effects and velocity-dependent activation and deactivation. J Exp Biol 201:1527–40. - PubMed
-
- Brown G, Wellings S. 1970. Electron microscopy of the skin of the teleost, Hippoglossoides elassodon. Z Zellforsch Mikrosk Anat 103:149–69. - PubMed
LinkOut - more resources
Full Text Sources
