This is a preprint.
T-cell receptor sequencing identifies prior SARS-CoV-2 infection and correlates with neutralizing antibody titers and disease severity
- PMID: 33791723
- PMCID: PMC8010755
- DOI: 10.1101/2021.03.19.21251426
T-cell receptor sequencing identifies prior SARS-CoV-2 infection and correlates with neutralizing antibody titers and disease severity
Update in
-
T cell receptor sequencing identifies prior SARS-CoV-2 infection and correlates with neutralizing antibodies and disease severity.JCI Insight. 2022 May 23;7(10):e150070. doi: 10.1172/jci.insight.150070. JCI Insight. 2022. PMID: 35439166 Free PMC article.
Abstract
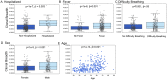
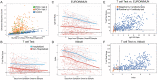
Measuring the adaptive immune response to SARS-CoV-2 can enable the assessment of past infection as well as protective immunity and the risk of reinfection. While neutralizing antibody (nAb) titers are one measure of protection, such assays are challenging to perform at a large scale and the longevity of the SARS-CoV-2 nAb response is not fully understood. Here, we apply a T-cell receptor (TCR) sequencing assay that can be performed on a small volume standard blood sample to assess the adaptive T-cell response to SARS-CoV-2 infection. Samples were collected from a cohort of 302 individuals recovered from COVID-19 up to 6 months after infection. Previously published findings in this cohort showed that two commercially available SARS-CoV-2 serologic assays correlate well with nAb testing. We demonstrate that the magnitude of the SARS-CoV-2-specific T-cell response strongly correlates with nAb titer, as well as clinical indicators of disease severity including hospitalization, fever, or difficulty breathing. While the depth and breadth of the T-cell response declines during convalescence, the T-cell signal remains well above background with high sensitivity up to at least 6 months following initial infection. Compared to serology tests detecting binding antibodies to SARS-CoV-2 spike and nucleoprotein, the overall sensitivity of the TCR-based assay across the entire cohort and all timepoints was approximately 5% greater for identifying prior SARS-CoV-2 infection. Notably, the improved performance of T-cell testing compared to serology was most apparent in recovered individuals who were not hospitalized and were sampled beyond 150 days of their initial illness, suggesting that antibody testing may have reduced sensitivity in individuals who experienced less severe COVID-19 illness and at later timepoints. Finally, T-cell testing was able to identify SARS-CoV-2 infection in 68% (55/81) of convalescent samples having nAb titers below the lower limit of detection, as well as 37% (13/35) of samples testing negative by all three antibody assays. These results demonstrate the utility of a TCR-based assay as a scalable, reliable measure of past SARS-CoV-2 infection across a spectrum of disease severity. Additionally, the TCR repertoire may be useful as a surrogate for protective immunity with additive clinical value beyond serologic or nAb testing methods.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
