The role of 14-3-3 proteins in cell signalling pathways and virus infection
- PMID: 33793048
- PMCID: PMC8093981
- DOI: 10.1111/jcmm.16490
The role of 14-3-3 proteins in cell signalling pathways and virus infection
Abstract
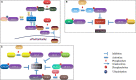
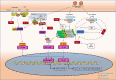
14-3-3 proteins are highly conserved in species ranging from yeast to mammals and regulate numerous signalling pathways via direct interactions with proteins carrying phosphorylated 14-3-3-binding motifs. Recent studies have shown that 14-3-3 proteins can also play a role in viral infections. This review summarizes the biological functions of 14-3-3 proteins in protein trafficking, cell-cycle control, apoptosis, autophagy and other cell signal transduction pathways, as well as the associated mechanisms. Recent findings regarding the role of 14-3-3 proteins in viral infection and innate immunity are also reviewed.
Keywords: 14-3-3 proteins; biological function; innate immunity; viral infection.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
-
- Chaudhri M, Scarabel M, Aitken A. Mammalian and yeast 14‐3‐3 isoforms form distinct patterns of dimers in vivo. Biochem Biophys Res Commun. 2003;300:679‐685. - PubMed
-
- Fu H, Subramanian RR, Masters SC. 14‐3‐3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617‐647. - PubMed
-
- Coblitz B, Wu M, Shikano S, Li M. C‐terminal binding: an expanded repertoire and function of 14‐3‐3 proteins. FEBS Lett. 2006;580:1531‐1535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

