A Proposed Mechanism for the Initial Myosin Binding Event on the Cardiac Thin Filament: A Metadynamics Study
- PMID: 33793247
- PMCID: PMC8080310
- DOI: 10.1021/acs.jpclett.1c00223
A Proposed Mechanism for the Initial Myosin Binding Event on the Cardiac Thin Filament: A Metadynamics Study
Abstract
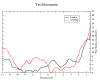
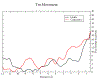
The movement of tropomyosin over filamentous actin regulates the cross-bridge cycle of the thick with thin filament of cardiac muscle by blocking and revealing myosin binding sites. Tropomyosin exists in three, distinct equilibrium states with one state blocking myosin-actin interactions (blocked position) and the remaining two allowing for weak (closed position) and strong myosin binding (open position). However, experimental information illuminating how myosin binds to the thin filament and influences tropomyosin's transition across the actin surface is lacking. Using metadynamics, we mimic the effect of a single myosin head binding by determining the work required to pull small segments of tropomyosin toward the open position in several distinct regions of the thin filament. We find differences in required work due to the influence of cardiac troponin T lead to preferential binding sites and determine the mechanism of further myosin head recruitment.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
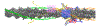

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

