Hemodynamic and electromechanical effects of paraquat in rat heart
- PMID: 33793552
- PMCID: PMC8016255
- DOI: 10.1371/journal.pone.0234591
Hemodynamic and electromechanical effects of paraquat in rat heart
Abstract
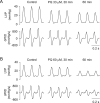
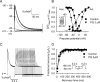
Paraquat (PQ) is a highly lethal herbicide. Ingestion of large quantities of PQ usually results in cardiovascular collapse and eventual mortality. Recent pieces of evidence indicate possible involvement of oxidative stress- and inflammation-related factors in PQ-induced cardiac toxicity. However, little information exists on the relationship between hemodynamic and cardiac electromechanical effects involved in acute PQ poisoning. The present study investigated the effects of acute PQ exposure on hemodynamics and electrocardiogram (ECG) in vivo, left ventricular (LV) pressure in isolated hearts, as well as contractile and intracellular Ca2+ properties and ionic currents in ventricular myocytes in a rat model. In anesthetized rats, intravenous PQ administration (100 or 180 mg/kg) induced dose-dependent decreases in heart rate, blood pressure, and cardiac contractility (LV +dP/dtmax). Furthermore, PQ administration prolonged the PR, QRS, QT, and rate-corrected QT (QTc) intervals. In Langendorff-perfused isolated hearts, PQ (33 or 60 μM) decreased LV pressure and contractility (LV +dP/dtmax). PQ (10-60 μM) reduced the amplitudes of Ca2+ transients and fractional cell shortening in a concentration-dependent manner in isolated ventricular myocytes. Moreover, whole-cell patch-clamp experiments demonstrated that PQ decreased the current amplitude and availability of the transient outward K+ channel (Ito) and altered its gating kinetics. These results suggest that PQ-induced cardiotoxicity results mainly from diminished Ca2+ transients and inhibited K+ channels in cardiomyocytes, which lead to LV contractile force suppression and QTc interval prolongation. These findings should provide novel cues to understand PQ-induced cardiac suppression and electrical disturbances and may aid in the development of new treatment modalities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

