Computational modelling of muscle fibre operating ranges in the hindlimb of a small ground bird (Eudromia elegans), with implications for modelling locomotion in extinct species
- PMID: 33793558
- PMCID: PMC8016346
- DOI: 10.1371/journal.pcbi.1008843
Computational modelling of muscle fibre operating ranges in the hindlimb of a small ground bird (Eudromia elegans), with implications for modelling locomotion in extinct species
Abstract
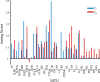
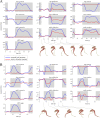
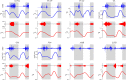
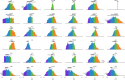
The arrangement and physiology of muscle fibres can strongly influence musculoskeletal function and whole-organismal performance. However, experimental investigation of muscle function during in vivo activity is typically limited to relatively few muscles in a given system. Computational models and simulations of the musculoskeletal system can partly overcome these limitations, by exploring the dynamics of muscles, tendons and other tissues in a robust and quantitative fashion. Here, a high-fidelity, 26-degree-of-freedom musculoskeletal model was developed of the hindlimb of a small ground bird, the elegant-crested tinamou (Eudromia elegans, ~550 g), including all the major muscles of the limb (36 actuators per leg). The model was integrated with biplanar fluoroscopy (XROMM) and forceplate data for walking and running, where dynamic optimization was used to estimate muscle excitations and fibre length changes throughout both gaits. Following this, a series of static simulations over the total range of physiological limb postures were performed, to circumscribe the bounds of possible variation in fibre length. During gait, fibre lengths for all muscles remained between 0.5 to 1.21 times optimal fibre length, but operated mostly on the ascending limb and plateau of the active force-length curve, a result that parallels previous experimental findings for birds, humans and other species. However, the ranges of fibre length varied considerably among individual muscles, especially when considered across the total possible range of joint excursion. Net length change of muscle-tendon units was mostly less than optimal fibre length, sometimes markedly so, suggesting that approaches that use muscle-tendon length change to estimate optimal fibre length in extinct species are likely underestimating this important parameter for many muscles. The results of this study clarify and broaden understanding of muscle function in extant animals, and can help refine approaches used to study extinct species.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Fibre operating lengths of human lower limb muscles during walking.Philos Trans R Soc Lond B Biol Sci. 2011 May 27;366(1570):1530-9. doi: 10.1098/rstb.2010.0345. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21502124 Free PMC article.
-
Musculoskeletal modelling of the Nile crocodile (Crocodylus niloticus) hindlimb: Effects of limb posture on leverage during terrestrial locomotion.J Anat. 2021 Aug;239(2):424-444. doi: 10.1111/joa.13431. Epub 2021 Mar 23. J Anat. 2021. PMID: 33754362 Free PMC article.
-
Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus).J Exp Biol. 2001 Aug;204(Pt 15):2717-31. doi: 10.1242/jeb.204.15.2717. J Exp Biol. 2001. PMID: 11533122
-
From fibre to function: are we accurately representing muscle architecture and performance?Biol Rev Camb Philos Soc. 2022 Aug;97(4):1640-1676. doi: 10.1111/brv.12856. Epub 2022 Apr 7. Biol Rev Camb Philos Soc. 2022. PMID: 35388613 Free PMC article. Review.
-
Scaling of avian bipedal locomotion reveals independent effects of body mass and leg posture on gait.J Exp Biol. 2018 May 22;221(Pt 10):jeb152538. doi: 10.1242/jeb.152538. J Exp Biol. 2018. PMID: 29789347 Review.
Cited by
-
Three-dimensional polygonal muscle modelling and line of action estimation in living and extinct taxa.Sci Rep. 2022 Mar 1;12(1):3358. doi: 10.1038/s41598-022-07074-x. Sci Rep. 2022. PMID: 35233027 Free PMC article.
-
Discrete element models for understanding the biomechanics of fossorial animals.Ecol Evol. 2022 Sep 16;12(9):e9331. doi: 10.1002/ece3.9331. eCollection 2022 Sep. Ecol Evol. 2022. PMID: 36177130 Free PMC article.
-
Modern three-dimensional digital methods for studying locomotor biomechanics in tetrapods.J Exp Biol. 2023 Apr 25;226(Suppl_1):jeb245132. doi: 10.1242/jeb.245132. Epub 2023 Feb 22. J Exp Biol. 2023. PMID: 36810943 Free PMC article. Review.
-
Testing the form-function paradigm: body shape correlates with kinematics but not energetics in selectively-bred birds.Commun Biol. 2024 Jul 24;7(1):900. doi: 10.1038/s42003-024-06592-w. Commun Biol. 2024. PMID: 39048787 Free PMC article.
-
A proposed standard for quantifying 3-D hindlimb joint poses in living and extinct archosaurs.J Anat. 2022 Jul;241(1):101-118. doi: 10.1111/joa.13635. Epub 2022 Feb 3. J Anat. 2022. PMID: 35118654 Free PMC article.
References
-
- McMahon TA (1984) Muscles, Reflexes, and Locomotion. Princeton: Princeton University Press.
-
- May MT (1968) Galen: On the Usefulness of the Parts of the Body. New York: Cornell University Press.
-
- Hill AV (1938) The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society of London, Series B 126: 136–195. - PubMed
-
- Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Critical Reviews in Biomedical Engineering 17: 359–410. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

