CD40 signaling augments IL-10 expression and the tolerogenicity of IL-10-induced regulatory dendritic cells
- PMID: 33793599
- PMCID: PMC8016274
- DOI: 10.1371/journal.pone.0248290
CD40 signaling augments IL-10 expression and the tolerogenicity of IL-10-induced regulatory dendritic cells
Abstract
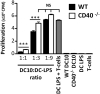
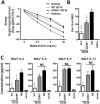
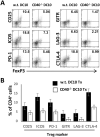
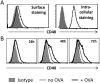
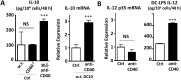
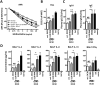
CD40 expressed on stimulatory dendritic cells (DC) provides an important accessory signal for induction of effector T cell responses. It is also expressed at lower levels on regulatory DC (DCreg), but there is little evidence that CD40 signaling contributes to the tolerogenic activity of these cells. Indeed, CD40 silencing within DCreg has been reported to induce T cell tolerance in multiple disease models, suggesting that CD40 is superfluous to DC-induced tolerance. We critically assessed whether CD40 does have a role in tolerance induced by IL-10-differentiated DC (DC10) by using DC10 generating from the bone marrow of wild-type (w.t.) or CD40-/- donor mice, or IL-10-complemented CD40-/- DC10 to treat asthmatic mice. Wild-type DC10 ablated the OVA-asthma phenotype via induction of Foxp3+ Treg responses, but CD40-/- DC10 had no discernible effects on primary facets of the phenotype (e.g., IL-5, IL-9, IL-13 levels, IgE & IgG1 antibodies; p>0.05) and were ≤40% effective in reversal of others. Foxp3+ T cells from the lungs of CD40-/- DC10-treated mice expressed reduced levels of a panel of six Treg-specific activation markers relative to Treg from w.t. DC10-treated mice. Coculture with effector T cells from asthmatic mice induced a marked upregulation of cell surface CD40 on w.t. DC10. While untreated CD40-/- and w.t. DC10 secreted equally low levels of IL-10, stimulation of w.t. DC10 with anti-CD40 for 72 h increased their expression of IL-10 by ≈250%, with no parallel induction of IL-12. Complementing IL-10 expression in CD40-/- DC10 by IL-10 mRNA transfection fully restored the cells' abilities to suppress the asthma phenotype. In summary, CD40 signaling in DC10 contributes importantly to their expression of IL-10 and to a robust induction of tolerance, including activation of induced Treg.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

