Comparison between magnetic resonance and ultrasound-derived indicators of hepatic steatosis in a pooled NAFLD cohort
- PMID: 33793651
- PMCID: PMC8016312
- DOI: 10.1371/journal.pone.0249491
Comparison between magnetic resonance and ultrasound-derived indicators of hepatic steatosis in a pooled NAFLD cohort
Abstract
Background & aims: MRI-based proton density fat fraction (PDFF) and the ultrasound-derived controlled attenuation parameter (CAP) are non-invasive techniques for quantifying liver fat, which can be used to assess steatosis in patients with non-alcoholic fatty liver disease (NAFLD). This study compared both of these techniques to histopathological graded steatosis for the assessment of fat levels in a large pooled NAFLD cohort.
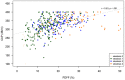
Methods: This retrospective study pooled N = 581 participants from two suspected NAFLD cohorts (mean age (SD) 56 (12.7), 60% females). Steatosis was graded according to NASH-CRN criteria. Liver fat was measured non-invasively using PDFF (with Liver MultiScan's Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation method, LMS-IDEAL, Perspectum, Oxford) and CAP (FibroScan, Echosens, France), and their diagnostic performances were compared.
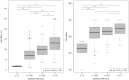
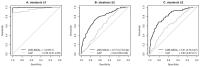
Results: LMS-IDEAL and CAP detected steatosis grade ≥ 1 with AUROCs of 1.00 (95% CI, 0.99-1.0) and 0.95 (95% CI, 0.91-0.99), respectively. LMS-IDEAL was superior to CAP for detecting steatosis grade ≥ 2 with AUROCs of 0.77 (95% CI, 0.73-0.82] and 0.60 (95% CI, 0.55-0.65), respectively. Similarly, LMS-IDEAL outperformed CAP for detecting steatosis grade ≥ 3 with AUROCs of 0.81 (95% CI, 0.76-0.87) and 0.63 (95% CI, 0.56-0.70), respectively.
Conclusion: LMS-IDEAL was able to diagnose individuals accurately across the spectrum of histological steatosis grades. CAP performed well in identifying individuals with lower levels of fat (steatosis grade ≥1); however, its diagnostic performance was inferior to LMS-IDEAL for higher levels of fat (steatosis grades ≥2 and ≥3).
Trial registration: ClinicalTrials.gov (NCT03551522); https://clinicaltrials.gov/ct2/show/NCT03551522. UMIN Clinical Trials Registry (UMIN000026145); https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000026145.
Conflict of interest statement
RB is the CEO and founder of Perspectum. AD, CB, CH, AA are employees of Perspectum. DK is employed by Texas Digestive Disease Consultants (GI Alliance). KI and AN are employees of Yokohoma City University Hospital. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

