Oral drug delivery systems using core-shell structure additive manufacturing technologies: a proof-of-concept study
- PMID: 33793804
- PMCID: PMC7940344
- DOI: 10.1093/jpp/rgaa037
Oral drug delivery systems using core-shell structure additive manufacturing technologies: a proof-of-concept study
Abstract
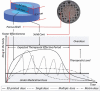
Objectives: The aim of this study was to couple fused deposition modelling 3D printing with melt extrusion technology to produce core-shell-structured controlled-release tablets with dual-mechanism drug-release performance in a simulated intestinal fluid medium. Coupling abovementioned technologies for personalized drug delivery can improve access to complex dosage formulations at a reasonable cost. Compared with traditional pharmaceutical manufacturing, this should facilitate the following: (1) the ability to manipulate drug release by adjusting structures, (2) enhanced solubility and bioavailability of poorly water-soluble drugs and (3) on-demand production of more complex structured dosages for personalized treatment.
Methods: Acetaminophen was the model drug and the extrusion process was evaluated by a series of physicochemical characterizations. The geometries, morphologies, and in vitro drug-release performances were compared between directly compressed and 3D-printed tablets.
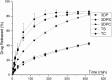
Key findings: Initially, 3D-printed tablets released acetaminophen more rapidly than directly compressed tablets. Drug release became constant and steady after a pre-determined time. Thus, rapid effectiveness was ensured by an initially fast acetaminophen release and an extended therapeutic effect was achieved by stabilizing drug release.
Conclusions: The favourable drug-release profiles of 3D-printed tablets demonstrated the advantage of coupling HME with 3D printing technology to produce personalized dosage formulations.
Keywords: 3D-printed tablets; acetaminophen; drug delivery systems; hot melt extrusion; oral delivery improvement; patient-focused dosages.
© The Author(s) 2021. Published by Oxford University Press on behalf of Royal Pharmaceutical Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets.Int J Pharm. 2017 Mar 15;519(1-2):186-197. doi: 10.1016/j.ijpharm.2016.12.049. Epub 2016 Dec 23. Int J Pharm. 2017. PMID: 28017768
-
Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation.Carbohydr Polym. 2017 Dec 1;177:49-57. doi: 10.1016/j.carbpol.2017.08.058. Epub 2017 Aug 18. Carbohydr Polym. 2017. PMID: 28962795 Free PMC article.
-
Development of 3D-printed dual-release fixed-dose combination through double-melt extrusion.Int J Pharm. 2024 Aug 15;661:124407. doi: 10.1016/j.ijpharm.2024.124407. Epub 2024 Jun 30. Int J Pharm. 2024. PMID: 38955239
-
Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance.Adv Drug Deliv Rev. 2021 May;172:52-63. doi: 10.1016/j.addr.2021.02.006. Epub 2021 Feb 9. Adv Drug Deliv Rev. 2021. PMID: 33571550 Free PMC article. Review.
-
Hot Melt Extrusion and its Application in 3D Printing of Pharmaceuticals.Curr Drug Deliv. 2021;18(4):387-407. doi: 10.2174/1567201817999201110193655. Curr Drug Deliv. 2021. PMID: 33176646 Review.
Cited by
-
Bile acid linked β-glucan nanoparticles for liver specific oral delivery of biologics.Biomater Sci. 2022 May 31;10(11):2929-2939. doi: 10.1039/d2bm00316c. Biomater Sci. 2022. PMID: 35471198 Free PMC article.
References
-
- Jain KK. Drug Delivery Systems. 1st ed.Totowa: Springer; 2008.
-
- Cui J, Yan Y, Wang Y, Caruso F. Templated assembly of pH‐labile polymer‐drug particles for intracellular drug delivery. Adv Funct Mater. 2012;22(22):4718–4723.doi: 10.1002/adfm.201201191 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

