Alternative splicing creates a pseudo-strictosidine β-d-glucosidase modulating alkaloid synthesis in Catharanthus roseus
- PMID: 33793899
- PMCID: PMC8133614
- DOI: 10.1093/plphys/kiaa075
Alternative splicing creates a pseudo-strictosidine β-d-glucosidase modulating alkaloid synthesis in Catharanthus roseus
Abstract
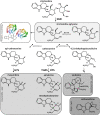
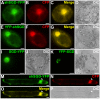
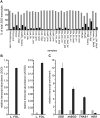
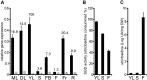
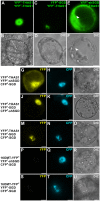
Deglycosylation is a key step in the activation of specialized metabolites involved in plant defense mechanisms. This reaction is notably catalyzed by β-glucosidases of the glycosyl hydrolase 1 (GH1) family such as strictosidine β-d-glucosidase (SGD) from Catharanthus roseus. SGD catalyzes the deglycosylation of strictosidine, forming a highly reactive aglycone involved in the synthesis of cytotoxic monoterpene indole alkaloids (MIAs) and in the crosslinking of aggressor proteins. By exploring C. roseus transcriptomic resources, we identified an alternative splicing event of the SGD gene leading to the formation of a shorter isoform of this enzyme (shSGD) that lacks the last 71-residues and whose transcript ratio with SGD ranges from 1.7% up to 42.8%, depending on organs and conditions. Whereas it completely lacks β-glucosidase activity, shSGD interacts with SGD and causes the disruption of SGD multimers. Such disorganization drastically inhibits SGD activity and impacts downstream MIA synthesis. In addition, shSGD disrupts the metabolic channeling of downstream biosynthetic steps by hampering the recruitment of tetrahydroalstonine synthase in cell nuclei. shSGD thus corresponds to a pseudo-enzyme acting as a regulator of MIA biosynthesis. These data shed light on a peculiar control mechanism of β-glucosidase multimerization, an organization common to many defensive GH1 members.
© American Society of Plant Biologists 2020. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Blanchard DJ, Cicek M, Chen J, Esen A (2001) Identification of β-glucosidase aggregating factor (BGAF) and mapping of BGAF binding regions on maize β-glucosidase. J Biol Chem 276:11895–11901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

