Gibberellin signaling mediates lateral root inhibition in response to K+-deprivation
- PMID: 33793923
- PMCID: PMC8133588
- DOI: 10.1093/plphys/kiaa093
Gibberellin signaling mediates lateral root inhibition in response to K+-deprivation
Abstract
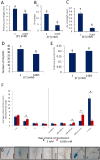
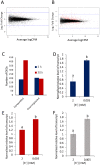
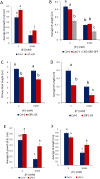
The potassium ion (K+) is vital for plant growth and development, and K+-deprivation leads to reduced crop yields. Here we describe phenotypic, transcriptomic, and mutant analyses to investigate the signaling mechanisms mediating root architectural changes in Arabidopsis (Arabidopsis thaliana) Columbia. We showed effects on root architecture are mediated through a reduction in cell division in the lateral root (LR) meristems, the rate of LR initiation is reduced but LR density is unaffected, and primary root growth is reduced only slightly. This was primarily regulated through gibberellic acid (GA) signaling, which leads to the accumulation of growth-inhibitory DELLA proteins. The short LR phenotype was rescued by exogenous application of GA but not of auxin or by the inhibition of ethylene signaling. RNA-seq analysis showed upregulation by K+-deprivation of the transcription factors JUNGBRUNNEN1 (JUB1) and the C-repeat-binding factor (CBF)/dehydration-responsive element-binding factor 1 regulon, which are known to regulate GA signaling and levels that regulate DELLAs. Transgenic overexpression of JUB1 and CBF1 enhanced responses to K+ stress. Attenuation of the reduced LR growth response occurred in mutants of the CBF1 target gene SFR6, implicating a role for JUB1, CBF1, and SFR6 in the regulation of LR growth in response to K+-deprivation via DELLAs. We propose this represents a mechanism to limit horizontal root growth in conditions where K+ is available deeper in the soil.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures





References
-
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311:91–94 - PubMed
-
- Achard P, Genschik P (2009) Releasing the brakes of plant growth: how GAs shutdown Della proteins. J Exp Bot 60:1085–1092 - PubMed
-
- Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P (2008b) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol 18:656–660 - PubMed
-
- Adams F (1971) Soil solution. InCarson EW, ed, The Plant Root and its Environment. Charlottesville, VA, University Press of Virginia, pp 441–481
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

