Sucrose promotes stem branching through cytokinin
- PMID: 33793932
- PMCID: PMC8133652
- DOI: 10.1093/plphys/kiab003
Sucrose promotes stem branching through cytokinin
Abstract
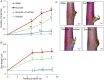
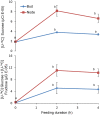
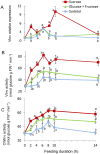
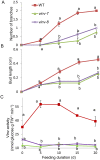
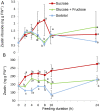
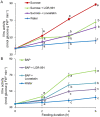
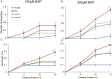
Shoot branching is an important aspect of plant architecture because it substantially affects plant biology and agricultural performance. Sugars play an important role in the induction of shoot branching in several species, including potato (Solanum tuberosum L.). However, the mechanism by which sugars affect shoot branching remains mostly unknown. In the present study, we addressed this question using sugar-mediated induction of bud outgrowth in potato stems under etiolated conditions. Our results indicate that sucrose feeding to detached stems promotes the accumulation of cytokinin (CK), as well as the expression of vacuolar invertase (VInv), an enzyme that contributes to sugar sink strength. These effects of sucrose were suppressed by CK synthesis and perception inhibitors, while CK supplied to detached stems induced bud outgrowth and VInv activity in the absence of sucrose. CK-induced bud outgrowth was suppressed in vinv mutants, which we generated by genome editing. Altogether, our results identify a branching-promoting module, and suggest that sugar-induced lateral bud outgrowth is in part promoted by the induction of CK-mediated VInv activity.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


Comment in
-
Branching out: new insights into sucrose-induced branching.Plant Physiol. 2021 Apr 23;185(4):1479-1480. doi: 10.1093/plphys/kiab041. Plant Physiol. 2021. PMID: 33893819 Free PMC article. No abstract available.
References
-
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 - PubMed
-
- Arrom L, Munné-Bosch S (2012) Sucrose accelerates flower opening and delays senescence through a hormonal effect in cut lily flowers. Plant Sci 188: 41–47 - PubMed
-
- Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24: 220–236 - PubMed
-
- Barbier FF, Lunn JE, Beveridge CA (2015b) Ready, steady, go! A sugar hit starts the race to shoot branching. Curr Opin Plant Biol 25: 39–45 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

