The role of GDP-l-galactose phosphorylase in the control of ascorbate biosynthesis
- PMID: 33793952
- PMCID: PMC8133566
- DOI: 10.1093/plphys/kiab010
The role of GDP-l-galactose phosphorylase in the control of ascorbate biosynthesis
Abstract
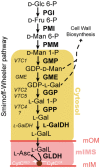
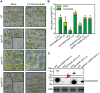
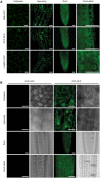
The enzymes involved in l-ascorbate biosynthesis in photosynthetic organisms (the Smirnoff-Wheeler [SW] pathway) are well established. Here, we analyzed their subcellular localizations and potential physical interactions and assessed their role in the control of ascorbate synthesis. Transient expression of C terminal-tagged fusions of SW genes in Nicotiana benthamiana and Arabidopsis thaliana mutants complemented with genomic constructs showed that while GDP-d-mannose epimerase is cytosolic, all the enzymes from GDP-d-mannose pyrophosphorylase (GMP) to l-galactose dehydrogenase (l-GalDH) show a dual cytosolic/nuclear localization. All transgenic lines expressing functional SW protein green fluorescent protein fusions driven by their endogenous promoters showed a high accumulation of the fusion proteins, with the exception of those lines expressing GDP-l-galactose phosphorylase (GGP) protein, which had very low abundance. Transient expression of individual or combinations of SW pathway enzymes in N. benthamiana only increased ascorbate concentration if GGP was included. Although we did not detect direct interaction between the different enzymes of the pathway using yeast-two hybrid analysis, consecutive SW enzymes, as well as the first and last enzymes (GMP and l-GalDH) associated in coimmunoprecipitation studies. This association was supported by gel filtration chromatography, showing the presence of SW proteins in high-molecular weight fractions. Finally, metabolic control analysis incorporating known kinetic characteristics showed that previously reported feedback repression at the GGP step, combined with its relatively low abundance, confers a high-flux control coefficient and rationalizes why manipulation of other enzymes has little effect on ascorbate concentration.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures





References
-
- Ali B, Pantha S, Acharya R, Ueda Y, Wu LB, Ashrafuzzaman M, Ishizaki T, Wissuwa M, Bulley S, Frei M (2019) Enhanced ascorbate level improves multi-stress tolerance in a widely grown indica rice variety without compromising its agronomic characteristics. J Plant Physiol 240: 1–9 - PubMed
-
- Amorim-Silva V, García-Moreno Á, Castillo AG, Lakhssassi N, Del Valle AE, Pérez-Sancho J, Li Y, Posé D, Pérez-Rodriguez J, Lin J, et al. (2019) TTL proteins scaffold brassinosteroid signaling components at the plasma membrane to optimize signal transduction in arabidopsis. Plant Cell 31: 1807–1828 - PMC - PubMed
-
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

